Selegiline
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /səˈlɛdʒɪliːn/ sə-LEJ-i-leen ("seh-LEH-ji-leen")[1][2] |
| Trade names | Eldepryl, Jumex, Zelapar, Emsam, others[3] |
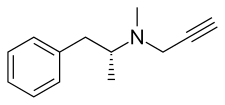
| Other names | L-Deprenyl; (R)-(–)-N,α-Dimethyl-N-2-propynylphenethylamine; (R)-(–)-N-Methyl-N-2-propynylamphetamine; (R)-(–)-N-2-Propynylmethamphetamine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697046 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | • Oral (tablet, capsule)[4][5] • Buccal (ODT)[6][7] • Transdermal (patch)[8][9] |
| Drug class | Monoamine oxidase inhibitor; Catecholaminergic activity enhancer; Norepinephrine releasing agent; Antiparkinsonian; Antidepressant; Neuroprotective |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: 4–10%[5][11][12] ODT: ~5–8× oral[13][7][14] Patch: 75%[9] |
| Protein binding | 85–90%[9][8][6] |
| Metabolism | Liver, other tissues (CYP2B6, CYP2C19, others)[5][18][9][19] |
| Metabolites | • Desmethylselegiline (DMS) • Levomethamphetamine (L-MA) • Levoamphetamine (L-A) |
| Elimination half-life | Oral: • S (single): 1.2–3.5 h[5] • S (multi): 7.7–9.7 h[5][12] • DMS (single): 2.2–3.8 h[5] • DMS (multi): 9.5 h[5] • L-MA: 14–21 h[5][7] • L-A: 16–18 h[5][7] ODT: • S (single): 1.3 h[6] • S (multi): 10 h[6] Patch: • S: 20 h[12][8] |
| Excretion | Urine (87%):[15][16][7][5][17] • L-MA: 20–63% • L-A: 9–26% • DMS: 1% • S: 0.01–0.03% Feces: 15%[15][7] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.109.269 |
| Chemical and physical data | |
| Formula | C13H17N |
| Molar mass | 187.286 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Selegiline, also known as L-deprenyl and sold under the brand names Eldepryl, Zelapar, and Emsam among others, is a medication which is used in the treatment of Parkinson's disease and major depressive disorder.[4][6][8][3] It has also been studied for a variety of other indications, but has not been formally approved for any other use.[20][21] The medication in the form licensed for depression has modest effectiveness for this condition that is similar to that of other antidepressants.[21][22][23] Selegiline is provided as a swallowed tablet or capsule[4][5] or an orally disintegrating tablet (ODT)[6][7] for Parkinson's disease and as a patch applied to skin for depression.[8][9]
Side effects of selegiline occurring more often than with placebo include insomnia, dry mouth, dizziness, nervousness, abnormal dreams, and application site reactions with the patch form, among others.[21][22][24][4][8] At high doses, selegiline has the potential for dangerous food and drug interactions, such as the tyramine-related "cheese reaction" or hypertensive crisis and risk of serotonin syndrome.[9][25][5] However, doses within the approved clinical range appear to have little to no risk of these interactions.[9][25][5] In addition, the ODT and transdermal patch forms of selegiline have reduced risks of such interactions compared to the conventional oral form.[7][9] Selegiline has no known misuse potential or dependence liability.[26][27][28][29]
Selegiline acts as a monoamine oxidase inhibitor (MAOI) and thereby increases levels of monoamine neurotransmitters in the brain.[30][11][25][5] At typical clinical doses used for Parkinson's disease, selegiline is a selective and irreversible inhibitor of monoamine oxidase B (MAO-B), increasing brain levels of dopamine.[30][11][25][5] At higher doses, it loses its specificity for MAO-B and also inhibits monoamine oxidase A (MAO-A), which increases serotonin and norepinephrine levels in the brain as well.[30][11][25][5] In addition to its MAOI activity, selegiline is a catecholaminergic activity enhancer (CAE) and enhances the impulse-mediated release of dopamine and norepinephrine in the brain.[31][32][33][34][25] This action may be mediated by TAAR1 agonism.[35][36] Selegiline is also a prodrug of levomethamphetamine and levoamphetamine in small amounts, which act as norepinephrine releasing agents (NRAs) and might be involved in its effects and side effects as well.[37][28][37][38] The levels of these metabolites are much lower with the ODT and transdermal patch forms of selegiline.[7][9] Chemically, selegiline is a substituted amphetamine,[39] a derivative of methamphetamine,[39] and the purified levorotatory enantiomer of deprenyl (the racemic form).[40][20]
Deprenyl was discovered and studied in the early 1960s.[40][20] Subsequently, selegiline was purified from deprenyl and was studied and developed itself.[40] Selegiline was first introduced for medical use in Hungary in 1977.[41] It was subsequently approved in the United Kingdom in 1982 and in the United States in 1989.[41][42] The ODT was approved in the United States in 2006 and in the European Union in 2010, while the patch was introduced in the United States in 2006.[41][20] In addition to its medical use, there has been interest in selegiline as a potential anti-aging drug and as a nootropic or "smart drug".[43] However, effects of this sort are controversial and uncertain.[44][45][46][47] Generic versions of selegiline are available in the case of the conventional oral form but not in the case of the ODT or transdermal patch forms.[48][49]
Medical uses[edit]
Parkinson's disease[edit]
In its oral and ODT forms, selegiline is used to treat symptoms of Parkinson's disease (PD).[4][6] It is most often used as an adjunct to drugs such as levodopa (L-DOPA), although it has been used off-label as a monotherapy.[50][51] The rationale for adding selegiline to levodopa is to decrease the required dose of levodopa and thus reduce the motor complications of levodopa therapy.[52] Selegiline delays the point when levodopa treatment becomes necessary from about 11 months to about 18 months after diagnosis.[53] There is some evidence that selegiline acts as a neuroprotective and reduces the rate of disease progression, though this is disputed.[51][52] In addition to parkinsonism, selegiline can improve symptoms of depression in people with Parkinson's disease.[54][55] There is evidence that selegiline may be more effective than rasagiline in the treatment of Parkinson's disease, which may be due to pharmacological differences between the drugs.[20][35][56]
Depression[edit]
Selegiline is used as an antidepressant in the treatment of major depressive disorder (MDD).[8][21] Both the oral selegiline and transdermal selegiline patch formulations are used in the treatment of depression.[21] However, oral selegiline is not approved for depression and is used off-label for this indication, while the transdermal patch is specifically licensed for treatment of depression.[4][8] Both standard clinical doses of oral selegiline (up to 10 mg/day) and higher doses of oral selegiline (e.g., 30 to 60 mg/day) have been used to treat depression, with the lower doses selectively inhibiting MAO-B and the higher doses producing dual inhibition of both MAO-A and MAO-B.[9][21] Unlike oral selegiline, transdermal selegiline bypasses first-pass metabolism, thereby avoiding inhibition of gastrointestinal and hepatic MAO-A and minimizing the risk of food and drug interactions whilst still allowing for selegiline to reach the brain and inhibit MAO-B.[9]
A 2023 systematic review and meta-analysis evaluated the effectiveness and safety of selegiline in the treatment of psychiatric disorders including depression.[21] It included both randomized and non-randomized published clinical studies.[21] The meta-analysis found that selegiline was more effective than placebo in terms of reduction in depressive symptoms (SMD = −0.96, k = 10, n = 1,308), response rates for depression improvement (RR = 1.61, k = 9, n = 1,238), and response rates for improvement of depression with atypical features (RR = 2.23, k = 3, n = 136).[21] Oral selegiline was significantly more effective than the selegiline patch in terms of depressive symptom improvement (SMD = −1.49, k = 6, n = 282 vs. SMD = −0.27, k = 4, n = 1,026, respectively; p = 0.03).[21] However, this was largely due to older and less methodologically rigorous trials at high risk for bias.[21] Oral selegiline studies also often employed much higher doses than usual, for instance 20 to 60 mg/day.[21] The quality of evidence of selegiline for depression was rated as very low overall, very low for oral selegiline, and low to moderate for transdermal selegiline.[21] For comparison, meta-analyses of other antidepressants for depression have found a mean effect size of about 0.3 (a small effect),[23][57] similar to that with transdermal selegiline.[21]
In two pivotal regulatory clinical trials of 6 to 8 weeks duration, selegiline decreased scores on depression rating scales (specifically the 17- and -28-item HDRS) by 9.0 to 10.9 points, whereas placebo decreased scores by 6.5 to 8.6 points, giving placebo-subtracted differences attributable to selegiline of 2.4 to 2.5 points.[8] A 2013 quantitative review of transdermal selegiline for depression, which pooled the results of these two trials, found that the placebo-subtracted number needed to treat (NNT) was 11 in terms of depression response (>50% reduction in symptoms) and 9 in terms of remission of depression (score of ≤10 on the MADRS).[22] For comparison, other antidepressants, including fluoxetine, paroxetine, duloxetine, vilazodone, adjunctive aripiprazole, olanzapine/fluoxetine, and extended-release quetiapine, have NNTs ranging from 6 to 8 in terms of depression response and 7 to 14 in terms of depression remission.[22] On the basis of these results, it was concluded transdermal selegiline has similar effectiveness to other antidepressants.[22] NNTs are measures of effect size and indicate how many individuals would need to be treated in order to encounter one additional outcome of interest.[22] Lower NNTs are better, and NNTs corresponding to Cohen's d effect sizes have been defined as 2.3 for a large effect (d = 0.8), 3.6 for a medium effect (d = 0.5), and 8.9 for a small effect (d = 0.2).[22] The effectiveness of transdermal selegiline for depression relative to side effects and discontinuation was considered to be favorable.[22]
While several large regulatory clinical trials of transdermal selegiline versus placebo for depression have been conducted, there is a lack of trials comparing selegiline to other antidepressants.[49] Although multiple doses of transdermal selegiline were assessed, a dose–response relationship for depression was never established.[49] Transdermal selegiline has shown similar clinical effectiveness in the treatment of atypical depression relative to typical depression and in the treatment of anxious depression relative to non-anxious depression.[49][58]
Transdermal selegiline does not cause sexual dysfunction and may improve certain domains of sexual function, for instance sexual interest, maintaining interest during sex, and sexual satisfaction.[59] These benefits were apparent in women but not in men.[59] The lack of sexual dysfunction with transdermal selegiline is in contrast to many other antidepressants, such as the selective serotonin reuptake inhibitors (SSRIs) and serotonin–norepinephrine reuptake inhibitors (SNRIs), which are associated with high rates of sexual dysfunction.[60]
Transdermal selegiline patches have been underutilized in the treatment of depression compared to other antidepressants.[49] A variety of factors contributing to this underutilization have been identified.[49] One major factor is the very high cost of transdermal selegiline, which is often not covered by insurance and frequently proves to be prohibitive.[49] Conversely, other widely available antidepressants are much cheaper in comparison.[49]
Available forms[edit]
Selegiline is available in the following three pharmaceutical forms:[48]
- Oral tablets and capsules 5 mg (brand names Eldepryl, Jumex, and generics)[4][5][41]
- Orally disintegrating tablets (ODTs) 1.25 mg (brand names Zelapar)[6][7]
- Transdermal patches 6, 9, and 12 mg/24 hours (brand name Emsam)[8][9][12][24]
The transdermal patch form is also known as the "selegiline transdermal system" or "STS" and is applied once daily.[9][12][24]
Contraindications[edit]
Selegiline is contraindicated with serotonergic antidepressants including selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs), with serotonergic opioids like meperidine, tramadol, and methadone, with other monoamine oxidase inhibitors (MAOIs) such as linezolid, phenelzine, and tranylcypromine, and with dextromethorphan, St. John's wort, cyclobenzaprine, pentazocine, propoxyphene, and carbamazepine.[6][8][4] Combination of selegiline with serotonergic agents may cause serotonin syndrome, while combination of selegiline with adrenergic or sympathomimetic agents like ephedrine or amphetamines may cause hypertensive crisis.[6][8] Long washout periods are required before starting and stopping these medications with discontinuation or initiation of selegiline.[6][8][4]
Consumption of tyramine-rich foods can result in hypertensive crisis with selegiline, also known as the "cheese effect" or "cheese reaction" due to the high amounts of tyramine present in some cheeses.[6][11][61][62] Examples of other foods that may have high amounts of tyramine and similar substances include yeast products, chicken liver, snails, pickled herring, red wines, some beers, canned figs, broad means, chocolate, and cream products.[62]
The preceding drug and food contraindications are dependent on selegiline dose and route, and hence are not necessarily absolute contraindications.[4][6][5][7][9] While high oral doses of selegiline (≥20 mg/day) can cause such interactions, oral doses within the approved clinical range (≤10 mg/day) appear to have little to no risk of these interactions.[9][25][5] In addition, the ODT and transdermal forms of selegiline have reduced risks of such interactions compared to the conventional oral form.[7][9]
Selegiline is also contraindicated in children less than 12 years of age and in people with pheochromocytoma, both due to heightened risk of hypertensive crisis.[8] For all human uses and all forms, selegiline is pregnancy category C, meaning that studies in pregnant animals have shown adverse effects on the fetus but there are no adequate studies in humans.[4][8]
Side effects[edit]
Side effects of the tablet form in conjunction with levodopa include, in decreasing order of frequency, nausea, hallucinations, confusion, depression, loss of balance, insomnia, increased involuntary movements, agitation, slow or irregular heart rate, delusions, hypertension, new or increased angina pectoris, and syncope.[4] Most of the side effects are due to a high dopamine signaling, and can be alleviated by reducing the dose of levodopa.[3] Selegiline can also cause cardiovascular side effects such as orthostatic hypotension, hypertension, atrial fibrillation, and other types of cardiac arrhythmias.[63]
The main side effects of the patch form for depression include application-site reactions, insomnia, dry mouth, dizziness, nervousness, and abnormal dreams.[8][24] The selegiline patch carries a black box warning about a possible increased risk of suicide, especially for young people,[8] as do all antidepressants since 2007.[64]
Side effects of selegiline that have been identified as occurring significantly more often than with placebo in meta-analyses for psychiatric disorders have included dry mouth (RR = 1.58), insomnia (RR = 1.61, NNH = 19), and application site reactions with the transdermal form (RR = 1.81, NNH = 7).[21][22] No significant diarrhea, headache, dizziness, nausea, sexual dysfunction, or weight gain were apparent in these meta-analyses.[21][22]
Selegiline does not appear to increase mortality in people with Parkinson's disease.[65][66] However, it appears to worsen cognition in people with Parkinson's disease over time.[67] Conversely, rasagiline does not seem to do so and can enhance cognition.[67]
Rarely, selegiline has been reported to induce or exacerbate impulse control disorders, hypersexuality, and paraphilias in people with Parkinson's disease.[68][69][70][71][72][73] However, MAO-B inhibitors like selegiline causing impulse control disorders is uncommon and controversial.[68] Selegiline has also been reported to activate or worsen rapid eye movement (REM) sleep behavior disorder in some people with Parkinson's disease.[74][75][76]
Selegiline has shown no misuse potential in humans or monkeys.[26][27][28][77][78] Likewise, it has no dependence potential in rodents.[29] This is in spite of its amphetamine active metabolites, levomethamphetamine and levoamphetamine, and is in contrast to agents like dextroamphetamine and dextromethamphetamine.[27][28][29][77][78] However, selegiline can strongly potentiate the reinforcing effects of exogenous β-phenethylamine by inhibiting its MAO-B-mediated metabolism.[28] Misuse of the combination of selegiline and β-phenethylamine has been reported.[79][80]
Overdose[edit]
Little information is available about clinically significant selegiline overdose.[4] The drug has been studied clinically at doses as high as 60 mg/day orally,[81][21] 10 mg/day as an ODT,[7] and 12 mg/24 hours as a transdermal patch.[9] In addition, deprenyl (the racemic form) has been clinically studied at doses as large as 100 mg/day.[30] During clinical development of oral selegiline, some individuals who were exposed to doses of 600 mg developed severe hypotension and psychomotor agitation.[4][6] Overdose may result in non-selective inhibition of both MAO-A and MAO-B and may be similar to overdose of other non-selective monoamine oxidase inhibitors (MAOIs) like phenelzine, isocarboxazid, and tranylcypromine.[4][6] Serotonin syndrome, hypertensive crisis, and/or death may occur with overdose.[4][6][8] No specific antidote to selegiline overdose is available.[8]
Interactions[edit]
Serotonin syndrome and hypertensive crisis[edit]
Both the oral and patch forms of selegiline come with strong warnings against combining it with drugs that could produce serotonin syndrome, such as selective serotonin reuptake inhibitors (SSRIs) and the cough medicine dextromethorphan (DXM).[4][8][82] Selegiline in combination with the opioid analgesic pethidine is not recommended, as it can lead to severe adverse effects.[82] Several other synthetic opioids such as tramadol and methadone, as well as various triptans, are also contraindicated due to potential for serotonin syndrome.[83][84]
All three forms of selegiline carry warnings about food restrictions to avoid hypertensive crisis that are associated with MAOIs.[4][6][8] The patch form was created in part to overcome food restrictions; clinical trials showed that it was successful. Additionally, in post-marketing surveillance from April 2006 to October 2010, only 13 self-reports of possible hypertensive events or hypertension were made out of 29,141 exposures to the drug, and none were accompanied by objective clinical data.[22] The lowest dose of the patch method of delivery, 6 mg/24 hours, does not require any dietary restrictions.[85] Higher doses of the patch and oral formulations, whether in combination with the older non-selective MAOIs or in combination with the reversible MAO-A inhibitor (RIMA) moclobemide, require a low-tyramine diet.[82]
Cytochrome P450 inhibitors and inducers[edit]
The cytochrome P450 enzymes involved in the metabolism of selegiline have not been fully elucidated.[5][18] CYP2D6 and CYP2C19 metabolizer phenotypes did not significantly affect the pharmacokinetics of selegiline, suggesting that these enzymes are minimally involved in its metabolism and that inhibitors and inducers of these enzymes would not importantly affect its pharmacokinetics.[18][39][86] Likewise, the strong CYP3A4 and CYP3A5 inhibitor itraconazole has minimal impact on the pharmacokinetics of selegiline, suggesting lack of major involvement of this enzyme as well.[18][87][6] On the other hand, the anticonvulsant carbamazepine, which is known to act as a strong inducer of CYP3A enzymes,[88] has paradoxically been found to increase exposure to selegiline and its metabolites levomethamphetamine and levoamphetamine by approximately 2-fold (with selegiline used as the transdermal patch form).[8][9] One enzyme thought to be majorly involved in the metabolism of selegiline based on in-vitro studies is CYP2B6.[5][18][9][19] However, there are no clinical studies of different CYP2B6 metabolizer phenotypes or of CYP2B6 inhibitors or inducers on the pharmacokinetics of selegiline.[61] In addition to CYP2B6, CYP2A6 may be involved in the metabolism of selegiline to a lesser extent.[61][89]
Birth control pills containing ethinylestradiol and a progestin like gestodene or levonorgestrel have been found to increase peak levels and overall exposure to oral selegiline by 10- to 20-fold.[18][90][91] High levels of selegiline can lead to loss of MAO-B selectivity and inhibition of MAO-A as well.[18][91] This increases susceptibility to side effects and interactions of non-selective monoamine oxidase inhibitors (MAOIs), such as tyramine-induced hypertensive crisis and serotonin toxicity when combined with serotonergic medications.[18][91] However, this study had a small sample size of 4 individuals as well as other methodological limitations.[18][91] The precise mechanism underlying the interaction is unknown, but is likely related to cytochrome P450 inhibition and consequent inhibition of selegiline first-pass metabolism by ethinylestradiol.[18] In contrast to birth control pills containing ethinylestradiol, menopausal hormone therapy with estradiol and levonorgestrel did not modify peak levels of selegiline and only modestly increased overall exposure (+59%).[18][90][92] Hence, menopausal hormone therapy does not pose the same risk of interaction as ethinylestradiol-containing birth control pills when taken together with selegiline.[90][92]
Selegiline inhibition of cytochrome P450 enzymes[edit]
Selegiline has been reported to inhibit several cytochrome P450 enzymes, including CYP2D6, CYP3A4/5, CYP2C19, CYP2B6, and CYP2A6.[8][93] It is a mechanism-based inhibitor of CYP2B6 and has been said to "potently" or "strongly" inhibit this enzyme in vitro.[94][93][95][96] It may inhibit the metabolism of bupropion, a major CYP2B6 substrate, into its active metabolite hydroxybupropion.[94][93][95] However, a study predicted that inhibition of CYP2B6 by selegiline would non-significantly affect exposure to bupropion.[96] Selegiline has not been listed or described as a clinically significant CYP2B6 inhibitor by the Food and Drug Administration (FDA) as of 2023.[88][8] One small study observing three patients found that selegiline was safe and well-tolerated in combination with bupropion.[95][97] Selegiline is also a potent mechanism-based inhibitor of CYP2A6 and may increase exposure to nicotine.[98][99] By inhibiting cytochrome P450 enzymes like CYP2B6 and CYP1A2, selegiline may inhibit its own metabolism and thereby interact with itself.[99][100]
Other interactions[edit]
Selegiline, at an MAO-B-selective dosage, did not appear to modify the pharmacological effects or pharmacokinetics of intravenous methamphetamine in one study.[101][102] Conversely, selegiline, also at MAO-B-selective doses, has been found to reduce the physiological and euphoric subjective effects of cocaine whilst not affecting its pharmacokinetics in some studies but not in others.[103][104][105][106][107][108]
Dopamine antagonists like antipsychotics or metoclopramide, which block dopamine receptors and thereby antagonize the dopaminergic effects of selegiline, could potentially reduce the effectiveness of selegiline.[6]
Pharmacology[edit]
Pharmacodynamics[edit]
Monoamine oxidase inhibitor[edit]
Selegiline acts as an enzyme inhibitor of the enzyme monoamine oxidase (MAO) and hence is known as a monoamine oxidase inhibitor (MAOI).[30][11][25][5] There are two types of MAO, MAO-A and MAO-B.[30][11][25][5] MAO-A metabolizes the monoamine neurotransmitters serotonin, dopamine, and norepinephrine as well as trace amines like tyramine, whereas MAO-B metabolizes dopamine and the trace amine β-phenethylamine.[30][11][25][5] At lower concentrations and at typical clinical doses (≤10 mg/day), selegiline selectively inhibits MAO-B.[30][11][25][5] Conversely, at higher concentrations and doses (≥20 mg/day), selegiline additionally inhibits MAO-A.[30][11][25][5] By selectively inhibiting MAO-B, selegiline increases levels of dopamine in the brain and thereby increases dopaminergic neurotransmission.[30][11][25][5] At higher doses, by inhibiting both MAO-A and MAO-B, selegiline increases brain levels of serotonin, dopamine, and norepinephrine and thereby increases serotonergic, dopaminergic, and noradrenergic neurotransmission.[30][11][25][5] Selegiline is an irreversible mechanism-based inhibitor of MAO that acts by covalently binding to the active site of the protein and thereby disabling it.[30][11][25][5][63]
Selegiline is thought to exert its therapeutic effects in the treatment of the motor symptoms of Parkinson's disease by increasing dopamine levels in the substantia nigra pars compacta (SNpc) of the basal ganglia, which projects to the caudate nucleus and putamen of the striatum, thereby enhancing the signaling of the nigrostriatal pathway.[63][30][109][110][17] In addition to the nigrostriatal pathway, selegiline may also influence and potentiate other dopaminergic pathways and areas, including the mesolimbic pathway, mesocortical pathway, tuberoinfundibular pathway, and chemoreceptor trigger zone, which may also be involved in its effects as well as side effects.[111][112][113] Selegiline and other MAO-B inhibitors may additionally improve non-motor symptoms in Parkinson's disease, for instance depression and motivational deficits, by increasing dopamine levels.[63] Selegiline may have some disease-modifying neuroprotective effects in Parkinson's disease by inhibiting the MAO-B-mediated oxidation of dopamine into reactive oxygen species that damage dopaminergic neurons in the nigrostriatal pathway via oxidative stress.[114][63] However, the pathophysiology of Parkinson's disease is complex and multifacted, and MAO-B inhibitors may only slow the progression of the disease and do not halt it.[114][63]
Following a single 5 or 10 mg oral dose of selegiline, 86% to 90% of MAO-B activity in blood platelets was inhibited within 2 to 4 hours and 98% of activity was inhibited after 24 hours.[5][30] Inhibition of platelet MAO-B activity persisted at above 90% for 5 days and almost 14 days were required before activity returned to baseline.[5][30] A lower dose of selegiline of 1 mg/day also inhibits platelet MAO-B activity by 70 to 100%.[30][115] However, despite this potent and long-lasting inhibition, optimal effectiveness in Parkinson's disease requires a dosage of 10 mg/day and the effectiveness lasts only about 2 to 3 days with discontinuation of selegiline.[30][116] Based on the preceding findings, selegiline almost completely inhibits platelet MAO-B act a dosage of 10 mg/day.[7] It is assumed that peripheral and brain MAO-B are inhibited with selegiline to similar extents.[47][117][30] Accordingly, selegiline at an MAO-B-selective dosage of 10 mg/day has been found to inhibit brain MAO-B by more than 90% in postmortem individuals with Parkinson's disease.[13][37][118][119] This dosage of selegiline has been found in such individuals to produce increases in brain levels of dopamine of 23 to 350% and of β-phenethylamine of 1,200 to 3,400% depending on the brain area and the study.[25][30][120][118][121][122] Brain MAO-B levels recover slowly upon discontinuation of selegiline, with a half-time of brain MAO-B synthesis and recovery of approximately 40 days in humans.[25][119]
Selegiline is about 500 to 1,000 times more potent in inhibiting MAO-B than MAO-A in vitro and about 100 times more potent in vivo in rodents.[30][11][47] The clinical selectivity of selegiline for MAO-B is lost at doses of the drug above 20 mg/day.[30] In a study of post-mortem individuals who were on selegiline 10 mg/day, MAO-A activity in the brain was inhibited by 38 to 86%.[30][25] A more recent study using positron emission tomography (PET) imaging similarly found inhibition of brain MAO-A by 33 to 70% in humans.[41][123] However, while brain dopamine and β-phenethylamine levels are substantially increased at this dosage, brain levels of serotonin and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) remain unchanged.[25][30][120] It has been found in animal studies that brain MAO-A must be inhibited by nearly 85% before serotonin, norepinephrine, or dopamine levels increase and result in increased functional activity as well as accompanying behavioral changes.[25][124] Selegiline at an oral dosage of 10 mg/day does not cause the "cheese effect" as assessed by oral tyramine and β-phenethylamine challenge tests.[5] These findings indicate that selegiline does not importantly inhibit MAO-A at a dosage of 10 mg/day.[5] However, a dosage of 20 mg/day selegiline did increase the pressor effect of tyramine, indicating that doses this high and above can significantly inhibit MAO-A.[25] The "cheese reaction" is known to be specifically dependent on inhibition of intestinal MAO-A.[30][25]
Besides increasing brain dopamine levels via MAO-B inhibition, selegiline strongly increases endogenous levels of β-phenethylamine, a major substrate of MAO-B.[30] Levels in of β-phenethylamine in the brain are increased 10- to 30-fold and levels in urine are increased 20- to 90-fold.[30][120][125] β-Phenethylamine is normally present in small amounts in the brain and urine and has been referred to as "endogenous amphetamine".[30][126] Similarly to amphetamines, it induces the release of norepinephrine and dopamine and produces psychostimulant effects.[30] Selegiline also strongly increases levels of β-phenethylamine with exogenous administration of β-phenethylamine.[30] The increase in endogenous levels of β-phenethylamine with selegiline might be involved in its effects, for instance claimed "psychic energizing" and mood-lifting effects as well as its effectiveness in the treatment of Parkinson's disease.[127][43][128] In contrast to amphetamine psychostimulants however, selegiline has no misuse potential.[43]
Besides selegiline itself, its major metabolite desmethylselegiline is pharmacologically active.[37][129] Compared to selegiline, desmethylselegiline is 60-fold less potent in inhibiting MAO-B in vitro, but is only 3- to 6-fold less potent in vivo.[5][129] Although desmethylselegiline levels with selegiline therapy are low, selegiline and desmethylselegiline are highly potent MAO-B inhibitors due to the irreversible nature of their inhibition.[30] As such, desmethylselegiline may contribute significantly to the MAO-B inhibition with selegiline.[30]
Findings from a 2021 study suggest that MAO-A is solely or almost entirely responsible for the striatal metabolism of dopamine rather than MAO-B.[130][131][132] Conversely, MAO-B was found to regulate tonic GABA levels and to indirectly enhance dopaminergic neurotransmission.[130][131][132] These findings warrant a rethinking of the pharmacological actions of MAO-B inhibitors like selegiline in the treatment of Parkinson's disease.[130][131][132]
Catecholaminergic activity enhancer[edit]
Selegiline has been found to act as a catecholaminergic activity enhancer (CAE).[31][32][33] It selectively enhances the activity of noradrenergic and dopaminergic neurons and does not affect the activity of serotonergic neurons.[34][25] The CAE actions of selegiline are distinct from those of catecholamine releasing agents like amphetamines.[31][32][33] Conversely, the actions are shared with certain trace amines like β-phenethylamine and tryptamine.[133][134] Selegiline and other CAEs enhance only impulse propogation-mediated release of catecholamines.[31][33] In relation to this, they lack the misuse potential of amphetamines.[31][32] Selegiline is active as a CAE at far lower concentrations and doses than those at which it starts to inhibit the monoamine oxidases.[135][25] For example, selegiline given subcutaneously in rodents selectively inhibits MAO-B with a single dose of at least 0.2 mg/kg, whereas CAE effects are apparent for noradrenergic neurons at a dose of 0.01 mg/kg (+42% activity) and for dopaminergic neurons at a dose of 0.025 mg/kg (+17% activity).[25][note 1] Selegiline is presently the only registered pharmaceutical medication with CAE actions that lacks concomitant potent catecholamine releasing effects.[133]
Other monoamine activity enhancers (MAEs) besides selegiline, like phenylpropylaminopentane (PPAP) and benzofuranylpropylaminopentane (BPAP), have been developed.[33][133] PPAP was derived from selegiline (and by extension from β-phenethylamine), while BPAP was derived from tryptamine.[133] These compounds are more potent and selective in their MAE actions than selegiline.[133][134] In addition, BPAP is an activity enhancer of not only catecholaminergic neurons but also of serotonergic neurons.[34] Unlike selegiline, PPAP and BPAP lack the MAO inhibition and amphetamine metabolites of selegiline.[133]
The actions of MAEs including selegiline may be due to TAAR1 agonism.[136][35] TAAR1 agonists have been found to enhance the release of monoamine neurotransmitters like dopamine and serotonin analogously to MAEs,[137][138][35] trace amines like β-phenethylamine and tryptamine are known to act as both TAAR1 agonists and MAEs,[137][138] and the TAAR1 antagonist EPPTB has been shown to reverse the CAE effects of BPAP and selegiline.[136][35] However, it has yet to be determined whether MAEs like BPAP and selegiline actually directly bind to and activate the TAAR1.[36][35] In any case, selegiline's active metabolites levomethamphetamine and levoamphetamine have been confirmed to bind to and activate the TAAR1.[139][140][141] As with selegiline, levomethamphetamine is also a CAE, although it is about 10-fold less potent in this action than selegiline itself.[32][25]
In contrast to selegiline, rasagiline is devoid of CAE actions.[20][142] In fact, it actually inhibits the CAE effects of selegiline.[35] This may explain differences in effectiveness between selegiline and rasagiline in the treatment of Parkinson's disease.[20][35][56] According to József Knoll, one of the original developers of selegiline, the CAE effect of selegiline may be more important than MAO-B inhibition in terms of effectiveness for Parkinson's disease.[32] Rasagiline may act as a TAAR1 antagonist to mediate its anti-CAE effects.[35] However, as with selegiline, binding to and modulation of the TAAR1 by rasagiline still requires confirmation.[35]
TAAR1 agonists like ulotaront and ralmitaront are under investigation for treatment of a variety of psychiatric disorders, such as depression and schizophrenia.[143][144]
Selegiline has potent pro-sexual or aphrodisiac effects in male rodents.[30][145][146][147] The pro-sexual effects of selegiline are stronger than those of dopamine agonists like apomorphine and bromocriptine and high doses of amphetamine.[30][145][147] These effects are not shared with other MAO-B inhibitors or the MAO-A inhibitor clorgiline and hence do not appear to be related to MAO inhibition.[30][146] Instead, the CAE actions of selegiline have been implicated in the pro-sexual effects.[20][133] Although selegiline has shown potent pro-sexual effects in rodents, these effects were not subsequently confirmed in primates.[30][148] In humans, selegiline for depression shows minimal pro-sexual effects in men, though it did significantly enhance several areas of sexual function in women.[59]
Catecholamine releasing agent[edit]
Two of selegiline's major metabolites, levomethamphetamine and levoamphetamine, are pharmacologically active.[37][38] Levomethamphetamine and levoamphetamine are sympathomimetic agents that work by inducing the release of norepinephrine and dopamine.[37][38] However, whereas the psychostimulants dextromethamphetamine and dextroamphetamine are relatively balanced releasers of dopamine and norepinephrine, levomethamphetamine is about 15-fold more potent in releasing norepinephrine relative to dopamine in vitro.[149][38][150][151] Similarly, levomethamphetamine and levoamphetamine are 10 times less potent at stimulating locomotor activity (a measure of psychostimulant effect) than dextromethamphetamine and dextroamphetamine in rodents.[37][152] Hence, these agents act more as selective norepinephrine releasing agents than as dual releasers of norepinephrine and dopamine.[149][38][151][37][150] The involvement of these metabolites in the effects of selegiline is controversial.[28] Both beneficial and harmful effects of these metabolites have been postulated.[28] It is unknown whether selegiline's amphetamine metabolites are involved in its effectiveness in the treatment of Parkinson's disease.[30]
It has been said that the amphetamine metabolites of selegiline might improve fatigue, but could also produce cardiovascular side effects like increased heart rate and blood pressure and reportedly may be able to cause insomnia, euphoria, psychiatric disturbances, and psychosis.[37][7][17] It is unknown what concentrations of levomethamphetamine and levoamphetamine produce sympathomimetic and other effects in humans and whether such concentrations are achieved with selegiline therapy.[37] However, cardiovascular side effects of selegiline have been found clinically and have been attributed to its amphetamine metabolites.[153][47] For comparison, rasagiline, which lacks amphetamine metabolites, has shown fewer adverse effects in clinical studies.[153][47][154] Animal studies suggest that selegiline's amphetamine metabolites may indeed be involved in its effects, such as arousal, wakefulness, and locomotor activity.[155][156][128][157]
In clinical studies, levomethamphetamine at oral doses of 1 to 10 mg has been found not to affect subjective drug responses, heart rate, blood pressure, core temperature, electrocardiography, respiration rate, oxygen saturation, or other clinical parameters.[158][159] As such, doses of levomethamphetamine of less than or equal to 10 mg have no significant physiological or subjective effects.[158][159] However, higher doses of levomethamphetamine, for instance 0.25 to 0.5 mg/kg (mean doses of ~18–37 mg) intravenously, have been reported to produce significant pharmacological effects, including increased heart rate and blood pressure, increased respiration rate, and subjective effects like intoxication and drug liking.[158][151] On the other hand, in contrast to dextroamphetamine and dextromethamphetamine, levomethamphetamine also produces subjective "bad" or aversive drug effects.[150][151] As with levomethamphetamine, oral doses of levoamphetamine of 20 to 60 mg have been reported to produce significant effects, for instance on wakefulness and mood.[160][161][162][163] With a 10 mg oral dose of selegiline, about 2 to 6 mg levomethamphetamine and 1 to 3 mg levoamphetamine is excreted in urine.[7][5][17]
The amphetamine metabolites of selegiline being involved in its effectiveness in the treatment of Parkinson's disease has been deemed unlikely.[30] High doses of levoamphetamine, for instance 50 mg/day, have been reported to be slightly effective in the treatment of Parkinson's disease.[30][17][162] It has been postulated that amphetamines are limitedly effective for Parkinson's disease as there is inadequate presynaptic dopamine to be released in patients with the condition.[160][162] In any case, this effectiveness of high doses of levoamphetamine could not be relevant to selegiline, which is administered at a dose of 10 mg/day.[30] Accordingly, relatively low levels of levoamphetamine occur with selegiline.[30] In one clinical study, levels of the amphetamine metabolites of selegiline were manipulated and there were no changes in clinical symptoms of Parkinson's disease.[30][164] This led the researchers to conclude that the beneficial clinical effects of selegiline in Parkinson's disease were not due to its amphetamine metabolites.[30][164] It is possible that there could be some small synergistic beneficial effect of selegiline with its amphetamine metabolites, but this has been considered improbable.[30]
Dextromethamphetamine and dextroamphetamine are known to produce dopaminergic neurotoxicity at high doses.[28] Such toxicity would be especially unfavorable in the context of Parkinson's disease.[28] However, levomethamphetamine and levoamphetamine are much weaker releasers of dopamine than the corresponding dextrorotatory enantiomers and have differing actions.[38][28] The levels of levomethamphetamine and levoamphetamine with typical clinical doses of selegiline are far lower than the levels of the corresponding dextrorotatory enantiomers that are known to produce clear signs of neurotoxicity.[28] As such, dopaminergic neurotoxicity with selegiline due to its amphetamine metabolites and consequent aggravation of Parkinson's disease is unlikely.[28]
Newer formulations of selegiline, such as the ODT and transdermal patch forms, have been developed which strongly reduce formation of the amphetamine metabolites and their associated effects.[7][9] In addition, other MAO-B inhibitors that do not metabolize into amphetamines, like rasagiline and safinamide, have been developed and introduced.[37][165]
Dopaminergic neuroprotection[edit]
Starting around the age of 45, dopamine content in the caudate nucleus decreases at a rate of about 13% per decade, and this neurodegeneration extends to the nigrostriatal dopaminergic pathway in general.[20][32][166][110][167][168][169] This is a very high rate of neuronal decay relative to brain aging generally.[32] Similarly, age-related decay of mesolimbic dopaminergic neurons as well as noradrenergic neurons is substantially slower than in the nigrostriatal pathway.[32][168] Symptoms of Parkinson's disease are known to develop when the dopamine content of the caudate nucleus drops below 30% of the normal level.[32][166][167][110] Loss of striatal dopamine reaches a level of 40% in healthy people by the age of 75, whereas in people with Parkinson's disease, the loss is around 70% at diagnosis and more than 90% at death.[32] Only about 0.1% of the human population develops Parkinson's disease.[167][110][32] In these individuals, the nigrostriatal pathway deteriorates more rapidly and prematurely than usual, for instance at a rate of 30 to 90% loss of dopamine content per decade.[167][110] However, it is thought that if humans lived much longer than the average lifespan, everyone would eventually develop Parkinson's disease.[167][110] Besides the nigrostriatal pathway, there is also considerable, albeit lesser, loss of dopaminergic neurons in people with Parkinson's disease in other pathways and areas, like the mesolimbic and mesocortical pathways.[168] There is even substantial loss of dopamine in non-brain tissues, like the adrenal cortex and retina, implicating a generalized degeneration of the whole dopamine system.[168]
The progressive loss of dopaminergic neurons in the nigrostriatal pathway as well as other areas has implications not only for motor control and risk of Parkinson's disease but also for cognition, emotions, learning, sexual activity, and other processes.[20][32][168] Dopamine itself is thought to play a major role in this degeneration by metabolism into reactive oxygen species that damage dopaminergic neurons.[32] Age-related degeneration of nigrostriatal dopaminergic neurons is similar in rodents and humans.[32][166] Selegiline has been found to prevent the age-related morphological changes in the nigrostriatal pathway of rodents and to produce accompanying preservations of cognitive and sexual functions.[20][32][166] These protective effects may be mediated by actions of selegiline including MAO-B inhibition, its catetcholaminergic activity enhancer activity, and other actions.[20][32][166] According to József Knoll and Ildikó Miklya, two of the developers of selegiline, the drug may act as a neuroprotective and may be able to slow the rate of age-related dopaminergic neurodegeneration in humans.[20][32][135] Knoll has advocated for the widespread use of a low dose of selegiline (1 mg/day or 10–15 mg/week) in the healthy population for such purposes.[166][133][167][135] However, antiaging and anti-neurodegenerative effects of selegiline in humans have not been clearly demonstrated as of present and this theory remains to be demonstrated.[47][20]
Other actions[edit]
Selegiline has a weak dopamine releasing effect, weakly blocks dopamine receptors, and weakly inhibits the reuptake of norepinephrine.[30] However, these actions are all of very low potency and are of questionable clinical significance.[30] On the basis of positron emission tomography research, selegiline does not significantly inhibit the brain dopamine transporter in humans at clinical doses.[123] The ODT and patch formulations of selegiline were used in this study.[123]
Selegiline appears to activate σ1 receptors, having a relatively high affinity for these receptors of approximately 400 nM.[170][171]
Unlike some of the hydrazine MAOIs like phenelzine and isocarboxazid, selegiline does not inhibit semicarbazide-sensitive amine oxidase (SSAO; also known as primary amine oxidase (PrAO) or as diamine oxidase (DAO)) nor does it pose a risk of vitamin B6 deficiency.[61] As a result, selegiline does not have risks of the side effects of these actions.[61]
Selegiline has been reported to inhibit several cytochrome P450 enzymes, including CYP2D6, CYP3A4/5, CYP2C19, CYP2B6, and CYP2A6.[8][93]
Pharmacokinetics[edit]
Absorption[edit]
Selegiline has an oral bioavailability of about 4 to 10%.[5][11][12][172] The average time to peak levels of selegiline is 0.6 to 1.4 hours, with a range in one study of about 0.5 to 1.5 hours.[5]
The circulating levels of selegiline and its metabolites following a single 10 mg oral dose have been studied.[5] The metabolites of selegiline include desmethylselegiline, levomethamphetamine, and levoamphetamine.[5] The average peak concentrations of selegiline across several studies ranged from 0.84 ± 0.6 μg/L to 2.2 ± 1.2 μg/L and the AUC levels ranged from 1.26 ± 1.19 μg⋅h/L to 2.17 ± 2.59 μg⋅h/L.[5] In the case of desmethylselegiline, the time to peak has been reported to be 0.8 ± 0.2 hours, the peak levels were 7.84 ± 2.11 μg/L to 13.4 ± 3.2 μg/L, and the area-under-the-curve (AUC) levels were 15.05 ± 4.37 μg⋅h/L to 40.3 ± 10.7 μg⋅h/L.[5] For levomethamphetamine, the peak levels were 10.2 ± 1.5 μg/L and the AUC levels were 150.2 ± 21.6 μg⋅h/L, whereas for levoamphetamine, the peak levels were 3.6 ± 2.9 μg/L and the AUC levels were 61.7 ± 44.0 μg⋅h/L.[5] For comparison, following a single 10 mg oral dose of dextromethamphetamine or dextroamphetamine, peak levels of these agents have been reported to range from 14 to 90 μg/L and from 15 to 34 μg/L, respectively.[173] Levels of desmethylselegiline and levomethamphetamine are 10- to 20-fold higher than selegiline levels with oral selegiline therapy.[86]
With repeated administration of selegiline, there is an accumulation of selegiline and its metabolites.[5] With a dosage of 10 mg once a day or 5 mg twice daily, peak levels of selegiline were 1.59 ± 0.89 μg/L to 2.33 ± 1.76 μg/L and AUC levels of selegiline were 6.92 ± 5.39 μg⋅h/L to 7.84 ± 5.43 μg⋅h/L after 1 week of treatment.[5] This equated to a 1.9- to 2.6-fold accumulation in peak levels and a 3.6- to 5.5-fold accumulation in AUC levels.[5] The metabolites of selegiline accumulate to a smaller extent than selegiline.[5] The AUC levels of desmethylselegiline increased by 1.5-fold and the peak and AUC levels of levomethamphetamine and levoamphetamine increased by 2-fold following 1 week of treatment with selegiline.[5] Selegiline appears to inhibit its own metabolism and that of desmethylselegiline with continuous use.[99][100]
The oral bioavailability of selegiline increases when it is ingested together with a fatty meal, as the molecule is fat-soluble.[3][174] There is a 3-fold increase in peak levels of selegiline and a 5-fold increase in AUC levels when it is taken orally with food.[5] The elimination half-life of selegiline is unchanged when it is taken with food.[5] In contrast to selegiline itself, the pharmacokinetics of its metabolites, desmethylselegililne, levomethamphetamine, and levoamphetamine, are unchanged when selegiline is taken with food.[5]
Distribution[edit]
The apparent volume of distribution of selegiline is 1,854 ± 824 L.[5] Selegiline and its metabolites rapidly cross the blood–brain barrier and enter the brain, where they most concentrated at the thalamus, basal ganglia, midbrain, and cingulate gyrus.[51][8] Selegiline especially accumulates in brain areas with high MAO-B content, such as the thalamus, striatum, cortex, and brainstem.[25] Concentrations of selegiline's metabolites in cerebrospinal fluid (CSF) are similar to those in blood, suggesting that accumulation in the brain over peripheral tissues does not occur.[25]
No data were originally available on the plasma protein binding of selegiline.[5] It has been stated that the plasma protein binding of selegiline is 94%, but there is no actual evidence to support this figure.[5] Subsequent research found that its plasma protein binding is 85 to 90%.[9][8][6]
Metabolism[edit]
Selegiline is metabolized in the intestines, liver, and other tissues.[5][25] More than 90% of orally administered selegiline is metabolized prior to reaching the bloodstream due to strong first-pass metabolism.[7] Selegiline (L-N-propargylmethamphetamine) is metabolized by N-demethylation into levomethamphetamine and by N-depropargylation into desmethylselegiline (L-N-propargylamphetamine).[9][61] Subsequently, levomethamphetamine is further metabolized into levoamphetamine by N-demethylation and desmethylselegiline is further metabolized into levoamphetamine by N-depropargylation.[7][61] Levomethamphetamine, levoamphetamine, and desmethylselegiline constitute the three major or primary metabolites of selegiline.[9][5][25] No racemization occurs in the metabolism of selegiline or its metabolites; that is, the levorotatory enantiomers are not converted into the dextrorotatory enantiomers, such as D-deprenyl, dextromethamphetamine, or dextroamphetamine.[11] Following their formation, the amphetamine metabolites of selegiline are also metabolized via hydroxylation and then conjugation via glucuronidation.[61] Besides the preceding metabolites, selegiline-N-oxide and formaldehyde are also known to be formed.[175] More than 40 minor metabolites of selegiline have been either detected or proposed.[175] Due to the amphetamine metabolites of selegiline, people taking selegiline may test positive for "amphetamine" or "methamphetamine" on drug screening tests.[176][177]
The exact cytochrome P450 enzymes responsible for the metabolism of selegiline have not been fully elucidated.[18] CYP2B6, CYP2C9, and CYP3A are thought to be significantly involved in the metabolism of selegiline on the basis of in vitro studies.[9][19][39] Other cytochrome P450 enzymes, including CYP1A2, CYP2A6, CYP2C8, CYP2D6, CYP2C19, and CYP2E1, may also be involved.[9][11][19][39] One review concluded that CYP2B6 and CYP2C19 are the leading candidates in selegiline metabolism.[18] CYP2B6 is thought to N-demethylate selegiline into desmethylselegiline and CYP2B6 and CYP2C19 are thought to N-depropargylate selegiline into levomethamphetamine.[9][19] Additionally, CYP2B6 and CYP2C19 are thought to metabolize desmethylselegiline into levoamphetamine and CYP2B6 is thought to N-demethylate levomethamphetamine into levoamphetamine.[9][19] CYP2D6 and CYP2C19 metabolizer phenotypes did not significantly affect the pharmacokinetics of selegiline, suggesting that these enzymes are minimally involved in its metabolism.[18][39][86] However, although most pharmacokinetic variables were unaffected, AUC levels of levomethamphetamine were 46% higher in CYP2D6 poor metabolizers compared to extensive metabolizers and desmethylselegiline AUC levels were 68% in CYP2C19 poor metabolizers compared to extensive metabolizers.[39][86] As with CYP2D6 and CYP2C19, CYP3A4 and CYP3A5 are unlikely to be majorly involved in the metabolism of selegiline as the strong inhibitor itraconazole has minimal impact on its pharmacokinetics.[18][87][6]
Elimination[edit]
Selegiline administered orally is recovered 87% in urine and 15% in feces as the unchanged parent drug and its metabolites.[15][7][16] Of selegiline excreted in urine, 20 to 63% is excreted as levomethamphetamine, 9 to 26% as levoamphetamine, 1% as desmethylselegiline, and 0.01 to 0.03% at unchanged selegiline.[7][5][17] In the case of levomethamphetamine and levoamphetamine, with an oral dose of 10 mg selegiline, this would be amounts of about 2 to 6 mg levomethamphetamine and about 1 to 3 mg levoamphetamine.[7][17] The near-absence of unchanged excreted selegiline indicates that selegiline is essentially completely metabolized prior to its excretion.[5][7]
The average elimination half-life of selegiline after a single oral dose ranges from 1.2 hours to 1.9 hours across studies.[5] With repeated administration, the half-life of selegiline increases to 7.7 ± 12.6 hours to 9.6 ± 13.6 hours.[5] The elimination half-life of selegiline's metabolite, desmethylselegiline, has been reported to range from 2.2 ± 0.6 hours to 3.8 hours.[5] The half-lives of its metabolites levomethamphetamine and levoamphetamine have been reported to be 14 hours and 16 hours, respectively.[5] Following repeated administration, the half-life of desmethylselegiline increased from 3.8 hours with the first dose to 9.5 hours following 1 week of daily selegiline doses.[5] Selegiline is a known inhibitor of several cytochrome P450 enzymes, such as CYP2B6 and CYP2A6.[94][93][95][99] It appears to inhibit its own metabolism and the metabolism of its metabolite desmethylselegiline.[99][100]
The oral clearance of selegiline is 59.4 ± 43.7 L/min.[5] This is described as very high and as almost 30-fold higher than hepatic blood flow.[5] The renal clearance of selegiline is 0.0072 L/h and is very low compared to its oral clearance.[5] These findings suggest that selegiline is extensively metabolized not only by the liver but also by non-hepatic tissues.[5]
Orally disintegrating tablet[edit]
Selegiline as an orally disintegrating tablet (ODT) is absorbed primarily buccally instead of being swallowed orally.[6][14] It was found to have 5- to 8-fold higher bioavailability, more consistent blood levels, and to produce fewer amphetamine metabolites than the standard oral tablet form.[14][13] It achieves blood levels of selegiline at a dose of 1.25 mg/day that are similar to those with conventional oral selegiline at a dose of 10 mg/day.[7] In addition, there is an at least 90% reduction in metabolites of selegiline including desmethylselegiline, levomethamphetamine, and levoamphetamine with the ODT formulation of selegiline compared to conventional oral selegiline.[7] Hence, levels of these metabolites are 10-fold lower with the ODT formulation.[153] The levels of amphetamine metabolites with the ODT formulation have been regarded as negligible.[6] This formulation of selegiline retains selectivity for MAO-B over MAO-A and likewise does not cause the "cheese effect" with consumption of tyramine-rich foods.[7]
Transdermal patch[edit]
The selegiline transdermal patch is indicated for application to the upper torso, upper thigh, or the outer upper arm once every 24 hours.[8] With application, an average of 25 to 30% (range 10 to 14%) of the selegiline content of the patch is delivered systemically over 24 hours.[9][8] This equates to about 0.3 mg selegiline per cm2 over 24 hours.[9] The patch has approximately 75% bioavailability, compared to 4 to 10% with the conventional oral form.[9][12] Transdermal selegiline results in significantly higher exposure to selegiline and lower exposure to all metabolites compared to conventional oral selegiline.[9] Selegiline levels are 50-fold higher and exposure to its metabolites 70% lower with the transdermal patch compared to oral administration at equivalent doses.[9] These differences are due to extensive first-pass metabolism with the oral form and the bypassing and absence of the first pass with the patch form.[9][12] Selegiline absorption and levels have been found to be equivalent when applied to the upper torso versus the upper thigh.[8] The drug does not accumulate in skin and is not significantly metabolized in skin.[8]
Chemistry[edit]
Selegiline belongs to the phenethylamine and amphetamine chemical families. It is also known as L-deprenyl, as well as (R)-(–)-N,α-dimethyl-N-(2-propynyl)phenethylamine or (R)-(–)-N-methyl-N-2-propynylamphetamine. The compound is a derivative of levomethamphetamine (L-methamphetamine) with a propargyl group attached to the nitrogen atom. It is also known as N-propargyl-L-methamphetamine.[7] This detail is borrowed from pargyline, an older MAO-B inhibitor of the phenylalkylamine group.[33] Selegiline is the levorotatory enantiomer of the racemic mixture deprenyl. D-deprenyl is the dextrorotatory enantiomer. Another clinically used MAOI of the amphetamine class is tranylcypromine.
Synthesis[edit]
Selegiline is synthesized by the alkylation of levomethamphetamine using propargyl bromide.[61][178][179][180][181]

History[edit]
Following the discovery in 1952 that the tuberculosis drug iproniazid elevated the mood of people taking it, and the subsequent discovery that the effect was likely due to inhibition of monoamine oxidase (MAO) and elevation of monoamine neurotransmitters in the brain, many people and companies started trying to discover monoamine oxidase inhibitors (MAOIs) to use as antidepressants.[11][182] Deprenyl, the racemic form of selegiline, was synthesized and discovered by Zoltan Ecseri at the Chinoin Pharmaceutical Company (part of Sanofi since 1993) in Budapest, Hungary.[11][183] Chinoin received a patent on the drug in 1962 and the compound was first published in the scientific literature in English in 1965.[11][184] Chinoin researchers had been studying substituted amphetamines since 1960, and decided to try synthesizing amphetamines that acted as MAOIs.[62] It had been known that methamphetamine was a reversible inhibitor of MAO.[62] Deprenyl, also known as N-propargyl-N-methylamphetamine,[33] is closely related to and inspired by pargyline (N-propargyl-N-methylbenzylamine), another MAOIs that had been synthesized earlier.[11][62][185] Deprenyl was initially referred to by the chemical name phenylisopropylmethylpropinylamine and the developmental code name E-250.[11][184] Work on the biology and effects of E-250 in animals and humans was conducted by a group led by József Knoll at Semmelweis University, which was also in Budapest.[11]
Deprenyl is a racemic compound (a mixture of two isomers called enantiomers).[11][62] Further work determined that the levorotatory enantiomer was a more potent MAOI, which was published in 1967, and subsequent work was done with the single enantiomer L-deprenyl.[11][62][186][187] In 1968, it was discovered by Johnston that monoamine oxidase exists in multiple forms.[11][62][188] In 1971, Knoll showed that selegiline highly selectively inhibits the B-isoform of monoamine oxidase (MAO-B) and proposed that it is unlikely to cause the infamous "cheese effect" (hypertensive crisis resulting from consuming foods containing tyramine) that occurs with non-selective MAOIs.[11][62][189] The lack of potentiation of tyramine effect by deprenyl had previously been reported in 1966 and 1968 studies, but could not be mechanistically explained until after the existence of multiple forms of MAO was discovered.[11][62][190]
Deprenyl and selegiline were initially studied as antidepressants for treatment of depression.[133][184] Deprenyl was first found to be effective for depression from 1965 to 1967,[133][191][192] while selegiline was first found to be effective for depression in 1971 and this was further corroborated in 1980.[133][193][194] A 1984 study that combined selegiline with β-phenethylamine reported remarkably high effectiveness in the treatment of depression similar to that with electroconvulsive therapy (ECT).[133][195] However, selegiline in its original oral form was never further developed or approved for the treatment of depression.[133]
A few years after the discovery that selegiline was a selective MAO-B inhibitor, two Parkinson's disease researchers based in Vienna, Peter Riederer and Walther Birkmayer, realized that selegiline could be useful in Parkinson's disease. One of their colleagues, Moussa B. H. Youdim, visited Knoll in Budapest and took selegiline from him to Vienna. In 1975, Birkmayer's group published the first paper on the effect of selegiline in Parkinson's disease.[187][196]
In the 1970s, there was also speculation that selegiline could be useful as an anti-aging drug or aphrodisiac.[197]
Selegiline was first introduced for clinical use in Hungary in 1977.[41] It was approved in the oral pill form under the brand name Jumex to treat Parkinson's disease.[41] The drug was then introduced in the United Kingdom in 1982.[41] In 1987, Somerset Pharmaceuticals in New Jersey, which had acquired the rights to develop selegiline in the United States, filed a New Drug Application (NDA) with the Food and Drug Administration (FDA) to market the drug for Parkinson's disease in this country.[42] While the NDA was under review, Somerset was acquired in a joint venture by two generic drug companies, Mylan and Bolan Pharmaceuticals.[42] Selegiline was approved for Parkinson's disease by the FDA in 1989.[42]
Selegiline was first shown to metabolize into levomethamphetamine and levoamphetamine in humans in 1978.[28][198] The involvement of these metabolites in the effects and side effects of selegiline has remained controversial and unresolved in the decades afterwards.[28][37] In any case, concerns about these metabolites have contributed to the development of newer MAO-B inhibitors like rasagiline and safinamide that lack such metabolites.[37][165]
The catecholaminergic activity enhancer (CAE) effects of selegiline became well-characterized and distinctly named by 1994.[32][25][142][20][34][199][200][201] These effects had been observed much earlier, dating back to the 1960s and 1970s, but were not properly distinguished from the other actions of selegiline, like MAO-B inhibition, until the 1990s.[32][25][34] More potent, selective, and/or expansive monoamine activity enhancers (MAEs), like phenylpropylaminopentane (PPAP) and benzofuranylpropylaminopentane (BPAP), were derived from selegiline and other compounds and were first described in 1992 and 1999, respectively.[33][134][202][133] These drugs had been proposed for potential treatment of psychiatric disorders like depression as well as for Parkinson's disease and Alzheimer's disease, but were never developed or marketed.[203][34][134][204][133]

In the 1990s, J. Alexander Bodkin at McLean Hospital, an affiliate of Harvard Medical School, began a collaboration with Somerset to develop delivery of selegiline via a transdermal patch in order to avoid the well known dietary restrictions of MAOIs.[197][205][206] Somerset obtained FDA approval to market the patch for depression in 2006.[207] Similarly, the orally disintegrating tablet (ODT) form of selegiline, marketed under the brand name Zelapar, was approved for Parkinson's disease in the United States in 2006 and in the European Union in 2010.[41]
Binding to and agonism of the TAAR1 as the mechanism responsible for the MAE effects of selegiline and related MAEs like PPAP and BPAP was first suggested by 2007[36] and was first substantiated in 2022.[136][35] TAAR1 agonists like ulotaront and ralmitaront are under development for treatment of various psychiatric disorders as of 2023.[143][144]
Society and culture[edit]
Names[edit]
Selegiline is the generic name of the drug and its INN, BAN, and DCF, while selegiline hydrochloride is the USAN.[208][209][210] The word "selegiline" is pronounced /səˈlɛdʒɪliːn/ (sə-LEJ-i-leen) or as "seh-LEH-ji-leen".[1][2] Selegiline is also known as L-deprenyl, L-deprenil, L-deprenalin, L-deprenaline, L-phenylisopropylmethylpropinylamine, and L-E-250.[20][208][209][210][184] It should not be confused with the racemic form, deprenyl (E-250), or with the dextrorotatory enantiomer, D-deprenyl, which are distinct substances.[208][40][20]
Major brand names of selegiline include Eldepryl and Jumex (oral tablet and capsule), Zelapar (orally disintegrating tablet or ODT), and Emsam (transdermal patch).[3][210] The brand name "Emsam" was derived from the names of two children, Emily and Samuel, of one of the employees at Somerset Pharmaceuticals, the developer of Emsam.[211]
Generic forms[edit]
Generic forms of oral selegiline are available in the United States.[48] However, generic forms of the orally disintegrating tablet and the transdermal patch are not available in this country.[48][49] The latter formulations of selegiline are very expensive, and this can be prohibitive to their use.[49][212] There has been poor insurance coverage of the transdermal patch form for depression, with insurance companies often requiring patients to first fail to respond to one or two other antidepressants and to be responsible for larger copayments.[49] It is expected that generics of the transdermal patch will become available at some point in the future.[49]
Availability[edit]
Conventional oral selegiline (brand names Eldepryl, Jumex) is widely marketed throughout the world, including in over 60 countries.[3][210][20] Conversely, the selegiline transdermal patch (brand name Emsam) is only marketed in the United States while the selegiline orally disintegrating tablet (brand name Zelapar) is marketed in the United States, the United Kingdom, and the European Union.[3][41][20]
Notable users[edit]
David Pearce, a British transhumanist philosopher, wrote his self-published book-length internet manifesto The Hedonistic Imperative[213] six weeks after starting to take selegiline.[214]
Sam Bankman-Fried, the founder and former CEO of the FTX cryptocurrency exchange, is known to have used selegiline for depression in the form of the Emsam patch for at least 5 to 10 years.[215][216] He is also known to have simultaneously taken Adderall for treatment of attention deficit hyperactivity disorder (ADHD)[215][216] and to have possessed non-pharmaceutical adrafinil, a prodrug of modafinil.[217]
Fictional representations[edit]
In Gregg Hurwitz's novel Out of the Dark, selegiline (Emsam) and tyramine-containing food were used to assassinate the president of the United States.[218]
Internet vendors[edit]
Selegline in non-pharmaceutical form is sold on the Internet without a prescription by online vendors for uses such as purported cognitive enhancement and anti-aging effects.[219]
Presence in ecstasy[edit]
In his 1993 book E for Ecstasy examining the uses of the street drug ecstasy in the United Kingdom, the writer, activist, and ecstasy advocate Nicholas Saunders highlighted test results showing that certain consignments of the drug also contained selegiline.[220] Consignments of ecstasy known as "Strawberry" contained what Saunders described as a "potentially dangerous combination of ketamine, ephedrine and selegiline," as did a consignment of "Sitting Duck" Ecstasy tablets.[221]
Doping in sport[edit]
Selegline is on the World Anti-Doping Agency (WADA)'s list of prohibited substances.[222] It is classified as a stimulant in this list, along with various amphetamines, methylphenidate, adrenergic sympathomimetics, modafinil, and other agents.[222] A review of the pharmacology of WADA prohibited substances noted that although selegiline is classified as a stimulant in the WADA prohibited substances list and stimulants can enhance physical performance, selegiline was seemingly included in the list not because of any short-term stimulant effects of its own, but rather because it metabolizes into small amounts of levomethamphetamine and levoamphetamine and can produce false positives for amphetamines on drug tests.[222]
Non-medical use[edit]
Anti-aging and longevity[edit]
József Knoll and his team are credited with having developed selegiline. Although selegiline's development as a potential treatment for Parkinson's disease, Alzheimer's disease, and depression was headed by other teams, Knoll remained at the forefront of research into the potential longevity enhancing effects of selegiline up until his death in 2018.[223][224][225] Knoll published his 2012 book How Selegiline ((-)-Deprenyl) Slows Brain Aging wherein he claims that:[135]: 90
"In humans, maintenance from sexual maturity on (-)-deprenyl (1mg daily) is, for the time being, the most promising prophylactic treatment to fight against the age related decay of behavioral performances, prolonging life, and preventing or delaying the onset of age-related neurodegenerative diseases such as Parkinson's and Alzheimer's".
The mechanism of selegiline's longevity-promoting effect has been researched by several groups, including Knoll and his associates at Semmelweis University, Budapest.[20] The drug has been determined to be a catecholaminergic activity enhancer when present in minuscule concentrations far below those at which monoamine oxidase inhibitory activity can be observed, thereby potentiating the release of catecholamine neurotransmitters in response to stimuli. Knoll maintains that micro-doses of selegiline act as a synthetic analogue to a known or unknown trace amine in order to preserve the brain catecholaminergic system, which he perceives as integral to the organism's ability to function in an adaptive, goal-directed and motivated manner during advancing physical age:[135]: 70, 43
"[...] enhancer regulation in the catecholaminergic brain stem neurons play[s] a key role in controlling the uphill period of life and the transition from adolescence to adulthood. The results of our longevity studies support the hypothesis that quality and duration of life rests upon the inborn efficiency of the catcholaminergic brain machinery, i.e. a high performing, long-living individual has a more active, more slowly deteriorating catecholaminergic system than its low performing, shorter living peer. Thus, a better brain engine allows for a better performance and a longer lifespan."
"Since the catecholaminergic and serotonergic neurons in the brain stem are of key importance in ensuring that the mammalian organism works as a purposeful, motivated, goal-directed entity, it is hard to overestimate the significance of finding safe and efficient means to slow the decay of these systems with passing time. The conclusion that the maintenance on (-)-deprenyl that keeps the catecholaminergic neurnsn a higher activity level is a safe and efficient anti- aging therapy follows from the discovery of the enhancer regulation in the catecholaminergic neurons of the brain stem. From the finding that this regulation starts working on a high activity level after weaning and the enhanced activity subsists during the uphill period of life, until sexual hormones dampen the enhancer regulation in the catecholaminergic and serotonergic neurons in the brain stem, and this event signifies the transition from developmental longevity into postdevelopmental longevity, the downhill period of life."
Despite findings by Knoll that selegiline can prolong lifespan in rodents by 35% however, other studies have had conflicting findings and have even found increased mortality with selegiline in rodents.[47] In humans with Parkinson's disease, selegline has been associated with cardiovascular and psychiatric complications and has not been found to reduce mortality in long-term studies.[47] As such, the claimed anti-aging and longevity benefits of selegiline are controversial and uncertain.[47][46]
Nootropic or "smart drug"[edit]
Selegiline is considered by some to be a nootropic, otherwise known as a cognitive enhancer or "smart drug", both at clinical and sub-clinical dosages, and has been used off-label and non-medically to improve cognitive performance.[44][226] It is one of the most popular such agents.[44] Selegiline has been found to have neuroprotective activity against certain neurotoxins and to increase the production of several brain growth factors, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and glial cell line-derived neurotrophic factor (GDNF).[20] The drug has also been found in animal models to improve learning ability and to help preserve it during ischemia and aging.[227][228][229][110] Despite claims that selegiline and other claimed nootropics have cogintive-enhancing effects however, these effects are controversial and their benefits versus risks are uncertain.[44]
Research[edit]
Depression[edit]
Selegiline has been clinically studied in combination with oral L-phenylalanine or β-phenethylamine in the treatment of depression and was reported to be effective.[134][126][195][230][231] L-Phenylalanine is known to be metabolized into β-phenethylamine, selegiline is known to strongly inhibit the metabolism of β-phenethylamine, and β-phenethylamine has been implicated in having psychostimulant-like mood-lifting effects.[134][30][126]
Anxiety[edit]
A small clinical study found that oral selegiline (10 mg/day) reduced symptoms of social anxiety disorder.[12][21][232] The effectiveness was modest, with a reduction in social anxiety scores from baseline of 32% over 6 weeks of treatment.[12][21][232]
ADHD[edit]
Selegiline has been limitedly studied in the treatment of attention deficit hyperactivity disorder (ADHD) in children, adolescents, and adults.[21][233][234][235] In a small randomized trial of selegiline for treatment of ADHD in children, there were improvements in attention, hyperactivity, and learning/memory performance but not in impulsivity.[236] A small clinical randomized trial compared selegiline to methylphenidate, a first line treatment for ADHD, and reported equivalent efficacy as assessed by parent and teacher ratings.[237] In another small randomized controlled trial of selegiline for the treatment of adult ADHD, a high dose of the medication for 6 weeks was not significantly more effective than placebo in improving symptoms.[234][238][239] Selegiline in its transdermal patch form (brand name Emsam) has also been assessed in the treatment of ADHD in children and adolescents in a small open-label pilot study sponsored by the manufacturer in 2003.[12][240] However, there was a high rate of discontinuation and development was not further pursued.[12][240]
Motivational disorders[edit]
Selegiline has been found to increase effort expenditure and to reverse pharmacologically-induced motivational deficits in rodents.[241][242][243][244] In case reports and small clinical studies, selegiline has been reported to improve apathy in people with traumatic brain injury, stroke, and schizophrenia.[241][245][246] In accordance with the preceding findings, selegiline, along with other dopaminergic and activating agents, is a potentially promising treatment for disorders of diminished motivation, including apathy, abulia, and akinetic mutism.[242][247][246]
Addiction[edit]
Selegiline has been evaluated for smoking cessation both as a monotherapy and in combination with nicotine replacement therapy in five clinical studies.[248][249][21] However, it is limitedly or not effective for this use.[248][249][21] It was also evaluated for treatment of cocaine dependence in one study, but was similarly not effective.[250] Studies are mixed on whether selegiline, at MAO-B-selective doses, reduces the effects of cocaine in humans.[103][104][104][105][106][107][108] Selegiline, also at an MAO-B-selective dosage, did not modify or potentiate the pharmacological effects of intravenous methamphetamine in a small clinical study.[101][102]
Sexual dysfunction[edit]
Selegiline has been assessed for treatment of sexual dysfunction induced by antipsychotics in people with schizophrenia, but was not effective in a single small clinical study.[251][252] It also did not improve sexual function in men with depression, but did improve several domains of sexual function in women with depression.[59]
Psychosis[edit]
Selegiline has been studied as an adjunct to antipsychotics in the treatment of schizophrenia in four clinical studies.[21][253] However, it failed to significantly reduce positive or negative symptoms of schizophrenia in meta-analyses of these studies.[21][253]
Excessive sleepiness[edit]
Selegiline has been evaluated for the treatment of narcolepsy in three small clinical studies.[254] It was found to be effective in these studies.[254] Selegiline has also been evaluated for treatment of hypersomnia (excessive sleeping or sleepiness) in people with myotonic dystrophy, but was not effective in a single small clinical study.[255][254]
Periodic limb movement disorder[edit]
Selegiline has been studied in the treatment of periodic limb movement disorder (PLMD) in a single small open-label clinical study.[256][257][258] It was reported to be effective as assessed by polysomnography, reducing periodic limb movements during sleep by about 60%.[256][258] Selegiline has not been studied for the related condition restless legs syndrome (RLS) as of 2023.[256][257] The drug has not been studied well enough in PLMD or RLS to be widely used in their treatment.[256]
Tardive dyskinedia[edit]
Selegiline was studied in the treatment of antipsychotic-induced tardive dyskinesia in one small clinical study, but was ineffective.[259]
Dementia and stroke[edit]
Selegiline has also been used off-label as a palliative treatment for dementia in Alzheimer's disease.[51] However, its clinical effectiveness is limited or lacking for this use.[260][111][261][262] It was also ineffective in the treatment of Lewy body dementia.[263] Selegiline has been used to support motor rehabilitation in stroke recovery, but evidence for this use is inadequate and no recommendation can be made for or against it.[264]
Disorders of consciousness[edit]
Selegiline has been studied in patients with disorders of consciousness, such as minimally conscious state, persistent vegetative state, and persistent coma, in a small open-label clinical study.[265][266] It was found to be effective in enhancing arousal and promoting recovery of consciousness in some of these individuals.[265][266]
Neurotoxicity[edit]
Selegiline has been reported to protect against the damage caused by the potent dopaminergic and/or noradrenergic neurotoxins 6-hydroxydopamine (6-OHDA), N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4), and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in animals.[267][30][268][269][270][271] Conversely, selegiline is ineffective in protecting against the serotonergic and noradrenergic neurotoxin 5,7-dihydroxytryptamine (5,7-DHT).[30][272]
Selegiline has also been reported to protect against methylenedioxymethamphetamine (MDMA)-induced serotonergic neurotoxicity in rodents.[273][274][275][276][277] The serotonergic neurotoxicity of MDMA appears to be dependent on release of dopamine and its subsequent metabolism by MAO-B within serotonergic neurons into hydroxyl radicals, which is blocked by MAO-B inhibition.[273][274] Likewise, selegiline prevented the serotonergic neurotoxicity of a combination of methylenedioxyaminoindane (MDAI) and dextroamphetamine.[278][279]
Conversely, selegiline failed to reduce the serotonergic neurotoxicity caused by fenfluramine and either did not affect or potentiated the serotonergic neurotoxicity caused by para-chloroamphetamine (PCA).[269][280][281][282] In addition, findings are mixed and conflicting on whether selegiline prevents amphetamine- and methamphetamine-induced dopaminergic neurotoxicity in rodents.[283][284][285][286]
Although MAO-B-selective doses of selegiline protect against MDMA-induced serotonergic neurotoxicity in rodents, combination of amphetamines like MDMA with monoamine oxidase inhibitors, including selegiline, can produce serious complications, including serotonin syndrome, hypertensive crisis, and death.[287][288]
Veterinary use[edit]
In veterinary medicine, selegiline is sold under the brand name Anipryl (manufactured by Zoetis). It is used in dogs to treat canine cognitive dysfunction and, at higher doses, pituitary-dependent hyperadrenocorticism (PDH).[289][290] Canine cognitive dysfunction is a form of dementia that mimics Alzheimer's disease in humans. Geriatric dogs treated with selegiline show improvements in sleeping pattern, reduced incontinence, and increased activity level; most show improvements by one month.[291][292] Though it is labeled for dog use only, selegiline has been used off-label for geriatric cats with cognitive dysfunction.[293]
Selegiline's efficacy in treating pituitary-dependent hyperadrenocorticism has been disputed.[289] Theoretically, it works by increasing dopamine levels, which downregulates the release of ACTH, eventually leading to reduced levels of cortisol.[293] Some claim that selegiline is only effective at treating PDH caused by lesions in the anterior pituitary (which comprise most canine cases).[294] The greatest sign of improvement is lessening of abdominal distention.[291]
Side effects in dogs are uncommon, but they include vomiting, diarrhea, diminished hearing, salivation, decreased weight and behavioral changes such as hyperactivity, listlessness, disorientation, and repetitive motions.[290][294]
Selegiline does not appear to have a clinical effect on horses.[294]
Notes[edit]
- ^ Selegiline given subcutaneously to rodents selectively inhibits MAO-B with a single 0.2–2.0 mg/kg dose or a continuous 0.05 to 0.25 mg/kg dosage and substantially inhibits MAO-A at a continuous dosage of 1.0 mg/kg.[25] It also produces catecholaminergic activity enhancer (CAE) effects with a subcutaneous dose of 0.01 mg/kg (+42% activity) for noradrenergic neurons and at a dose of 0.025 mg/kg (+17% activity) for dopaminergic neurons.[25] For comparison, the dosage used in humans orally is around 1 mg per 10 kg or 0.1 mg/kg daily.[25]
References[edit]
- ^ a b "Drug Treatments for Parkinson's" (PDF). Retrieved July 5, 2024.
Selegiline (seh-LEH-ji-leen)
- ^ a b Acosta WR (2020). Pharmacology for Health Professionals. Jones & Bartlett Learning. p. 66. ISBN 978-1-284-24083-2. Retrieved July 5, 2024.
sell-eh'-geh-leen
- ^ a b c d e f g "Selegiline". Drugs.com. Archived from the original on July 3, 2024. Retrieved February 7, 2016.
- ^ a b c d e f g h i j k l m n o p q r "ELDEPRYL® (Selegiline Hydrochloride) Tablets, USP Label" (PDF). Food and Drug Administration. January 2008. Retrieved July 3, 2024.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn Mahmood I (August 1997). "Clinical pharmacokinetics and pharmacodynamics of selegiline. An update". Clin Pharmacokinet. 33 (2): 91–102. doi:10.2165/00003088-199733020-00002. PMID 9260033.
- ^ a b c d e f g h i j k l m n o p q r s t u v w "ZELAPAR® (Selegiline Hydrochloride) Orally Disintegrating Tablets" (PDF). Food and Drug Administration. July 2021. Retrieved July 3, 2024.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa Poston KL, Waters C (October 2007). "Zydis selegiline in the management of Parkinson's disease". Expert Opin Pharmacother. 8 (15): 2615–2624. doi:10.1517/14656566.8.15.2615. PMID 17931095.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae "EMSAM® (Selegiline Transdermal System) Label" (PDF). Food and Drug Administration. July 2017. Retrieved July 2, 2024.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag Lee KC, Chen JJ (November 2007). "Transdermal selegiline for the treatment of major depressive disorder". Neuropsychiatric Disease and Treatment. 3 (5): 527–537. doi:10.2147/ndt.s12160200 (inactive July 6, 2024). PMC 2656289. PMID 19300583.
{{cite journal}}: CS1 maint: DOI inactive as of July 2024 (link) - ^ Anvisa (March 31, 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published April 4, 2023). Archived from the original on August 3, 2023. Retrieved August 16, 2023.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab Magyar K (2011). "The Pharmacology of Selegiline" (PDF). In Youdim M, Riederer P (eds.). Monoamine Oxidases and Their Inhibitors. International Review of Neurobiology. Vol. 100. Academic Press. pp. 65–84. doi:10.1016/B978-0-12-386467-3.00004-2. ISBN 978-0-12-386467-3. PMID 21971003.
The hope to discover a clinically applicable antidepressant gave the impetus to the Chinoin Pharmaceutical Company in 1962 to synthesize a "me-too" drug on the name of E-250. This number indicated that deprenyl was the 250th in the series of compounds. The patent of deprenyl was issued in Hungary in 1962. [...] Nevertheless, deprenyl is an original Hungarian drug, synthesized by Z. Ecsery in Budapest and developed by Knoll and his group. It was synthesized as an antidepressant, psychostimulant agent, but its therapeutic application had been realized in totally different field, as it was originally planned. [...] In 1967 it was published that the levorotatory enantiomer of deprenyl is more potent inhibitor of MAO than the dextrorotatory form, and after 1967, all of the studies, including the clinical application of the drug, were conducted with the (–)-optical antipode of deprenyl, named selegiline (Magyar et al., 1967). [...] In 1968, on the basis of substrate specificity and inhibitor sensitivity, Johnston discoveredthattheMAO enzyme exists inmultiple forms, being sensitive or nottothe effect of clorgyline, using the substrate of 5-HT.
- ^ a b c d e f g h i j k l Pae CU, Lim HK, Han C, Neena A, Lee C, Patkar AA (August 2007). "Selegiline transdermal system: current awareness and promise". Prog Neuropsychopharmacol Biol Psychiatry. 31 (6): 1153–1163. doi:10.1016/j.pnpbp.2007.04.020. PMID 17614182.
- ^ a b c Löhle M, Storch A (November 2008). "Orally disintegrating selegiline for the treatment of Parkinson's disease". Expert Opin Pharmacother. 9 (16): 2881–2891. doi:10.1517/14656566.9.16.2881. PMID 18937619.
In conclusion, these results indicate that 1.25 mg of orally disintegrating selegiline are capable of selectively blocking MAO-B activity to a similar extent as 10 mg of conventional selegiline, which at therapeutic doses inhibits MAO-B in the human brain by more than 90% [38].
- ^ a b c Clarke A, Brewer F, Johnson ES, Mallard N, Hartig F, Taylor S, Corn TH (November 2003). "A new formulation of selegiline: improved bioavailability and selectivity for MAO-B inhibition". Journal of Neural Transmission. 110 (11): 1241–1255. doi:10.1007/s00702-003-0036-4. PMID 14628189. S2CID 711419.
- ^ a b c Heinonen EH, Anttila MI, Lammintausta RA (December 1994). "Pharmacokinetic aspects of l-deprenyl (selegiline) and its metabolites". Clin Pharmacol Ther. 56 (6 Pt 2): 742–749. doi:10.1038/clpt.1994.204. PMID 7995016.
- ^ a b Heinonen EH, Myllylä V, Sotaniemi K, Lamintausta R, Salonen JS, Anttila M, Savijärvi M, Kotila M, Rinne UK (November 1989). "Pharmacokinetics and metabolism of selegiline". Acta Neurologica Scandinavica. Supplementum. 126: 93–99. doi:10.1111/j.1600-0404.1989.tb01788.x. PMID 2515726. S2CID 221440315.
- ^ a b c d e f g Chrisp P, Mammen GJ, Sorkin EM (May 1991). "Selegiline: A Review of its Pharmacology, Symptomatic Benefits and Protective Potential in Parkinson's Disease". Drugs Aging. 1 (3): 228–248. doi:10.2165/00002512-199101030-00006. PMID 1794016.
- ^ a b c d e f g h i j k l m n o Rodrigues AD (June 2022). "Drug Interactions Involving 17α-Ethinylestradiol: Considerations Beyond Cytochrome P450 3A Induction and Inhibition". Clin Pharmacol Ther. 111 (6): 1212–1221. doi:10.1002/cpt.2383. PMID 34342002.
Unfortunately, available in vitro data sets for selegiline are confusing and do not necessarily shed light on the observed DDI with EE-containing OC. Selegiline is metabolized to two major metabolites (desmethylselegiline and methamphetamine) and panels of recombinant proteins present CYP2B6 and CYP2C19 as leading candidates, although metabolism is also reported for other CYP forms.50-52 Importantly, clinical data do not support the CYP3A4/5-dependent metabolism of selegiline, since itraconazole has a minimal impact on its PK.53 Likewise, both CYP2D6 and CYP2C19 can be ruled out because selegiline PK is not significantly impacted in poor metabolizer subjects.54,55 Although liver CYP2B6 is described as a major selegiline-metabolizing CYP in vitro, its expression in gut is low (Table S1)56,57, and there are no reports describing the PK of selegiline in CYP2B6 genotyped subjects.
- ^ a b c d e f Hidestrand M, Oscarson M, Salonen JS, Nyman L, Pelkonen O, Turpeinen M, Ingelman-Sundberg M (November 2001). "CYP2B6 and CYP2C19 as the major enzymes responsible for the metabolism of selegiline, a drug used in the treatment of Parkinson's disease, as revealed from experiments with recombinant enzymes". Drug Metab Dispos. 29 (11): 1480–1484. PMID 11602525.
- ^ a b c d e f g h i j k l m n o p q r s t u Miklya I (November 2016). "The significance of selegiline/(-)-deprenyl after 50 years in research and therapy (1965-2015)". Molecular Psychiatry. 21 (11): 1499–1503. doi:10.1038/mp.2016.127. PMID 27480491.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x Rossano F, Caiazza C, Sobrino A, Solini N, Vellucci A, Zotti N, Fornaro M, Gillman K, Cattaneo CI, Van den Eynde V, Birkenhager TK, Ruhé HG, Stahl S, Iasevoli F, de Bartolomeis A (July 2023). "Efficacy and safety of selegiline across different psychiatric disorders: A systematic review and meta-analysis of oral and transdermal formulations". Eur Neuropsychopharmacol. 72: 60–78. doi:10.1016/j.euroneuro.2023.03.012. PMID 37087864.
- ^ a b c d e f g h i j k Citrome L, Goldberg JF, Portland KB (November 2013). "Placing transdermal selegiline for major depressive disorder into clinical context: number needed to treat, number needed to harm, and likelihood to be helped or harmed". Journal of Affective Disorders. 151 (2): 409–417. doi:10.1016/j.jad.2013.06.027. PMID 23890583.
- ^ a b Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JP, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JP, Geddes JR (April 2018). "Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis". Lancet. 391 (10128): 1357–1366. doi:10.1016/S0140-6736(17)32802-7. PMC 5889788. PMID 29477251.
- ^ a b c d Robinson DS, Amsterdam JD (January 2008). "The selegiline transdermal system in major depressive disorder: a systematic review of safety and tolerability". J Affect Disord. 105 (1–3): 15–23. doi:10.1016/j.jad.2007.04.024. PMID 17568687.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai Gerlach M, Youdim MB, Riederer P (December 1996). "Pharmacology of selegiline". Neurology. 47 (6 Suppl 3): S137–S145. doi:10.1212/wnl.47.6_suppl_3.137s. PMID 8959982.
In contrast, evidence drawn from postmortem studies27 with patients who had been on a daily dosage of 10 mg suggests that even though the oxidation of 5-HT (a preferred MAO-A substrate) was inhibited in brain homogenates of selegilinetreated patients with PD (38 to 84%, depending on the brain region), functional selectivity can nevertheless be maintained. Thus while DA and PE concentrations in the striatum are substantially increased above control values, by 350 and 1,300%, respectively,16,34 those of 5-HT and 5-hydroxyindole acetic acid are not altered.27 Green et al.35 showed that brain MAO-A has to be nearly 85% inhibited before brain 5-HT, NA, or DA concentrations increase and produce an increased functional activity.
- ^ a b Finberg JP, Rabey JM (2016). "Inhibitors of MAO-A and MAO-B in Psychiatry and Neurology". Front Pharmacol. 7: 340. doi:10.3389/fphar.2016.00340. PMC 5067815. PMID 27803666.
Clinical studies have not found evidence of abuse liability in humans (Yasar et al., 1996), and in addition it should be noted that selegiline does not induce addictive behavior in monkeys (Winger et al., 1994).
- ^ a b c Fabbrini G, Abbruzzese G, Marconi S, Zappia M (2012). "Selegiline: a reappraisal of its role in Parkinson disease". Clin Neuropharmacol. 35 (3): 134–140. doi:10.1097/WNF.0b013e318255838b. PMID 22592509.
As selegiline is metabolized to L-methamphetamine and L-amphetamine, there is a theoretical concern that it may have abuse potential. This has not been confirmed in animal models of amphetamine abuse; the peak concentrations of amphetamine and methamphetamine achieved during therapeutic dosing fall far below those likely to result in addiction or withdrawal effects, and experience from clinical practice confirms that selegiline has little or no abuse potential.9–11
- ^ a b c d e f g h i j k l m n Yasar S, Goldberg JP, Goldberg SR (January 1, 1996). "Are metabolites of l-deprenyl (Selegiline) useful or harmful? Indications from preclinical research". Deprenyl — Past and Future. Journal of Neural Transmission. Supplementum. Vol. 48. pp. 61–73. doi:10.1007/978-3-7091-7494-4_6. ISBN 978-3-211-82891-5. PMID 8988462.
- ^ a b c Nickel B, Szelenyi I, Schulze G (December 1994). "Evaluation of physical dependence liability of l-deprenyl (selegiline) in animals". Clin Pharmacol Ther. 56 (6 Pt 2): 757–767. doi:10.1038/clpt.1994.206. PMID 7995018.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av Heinonen EH, Lammintausta R (1991). "A review of the pharmacology of selegiline". Acta Neurologica Scandinavica. Supplementum. 136: 44–59. doi:10.1111/j.1600-0404.1991.tb05020.x. PMID 1686954.
In human brain, dopamine concentrations were about 40-50 % higher in parkinsonian patients treated with selegiline 10 mg daily than in those without it (8). [...] In human brain the concentrations of PEA were 2-6 ng/g after a 10 mg daily dose of selegiline, the highest concentrations being detected in the striatum, thalamus and also red nucleus (95). In striatum and limbic areas the increases of PEA concentration in parkinsonian brain have been over 1000 and 3000 % respectively (57). In healthy volunteers, selegiline treatment caused a 20 to 90-fold increase in PEA excretion as compared to their control values (22).
- ^ a b c d e Knoll J (1997). "Istoriia deprenil--pervogo selektivnogo ingibitora monoaminoksidazy tipa B" [History of deprenyl--the first selective inhibitor of monoamine oxidase type B]. Voprosy Meditsinskoi Khimii. 43 (6): 482–493. PMID 9503565.
- ^ a b c d e f g h i j k l m n o p q r s t Knoll J (February 1998). "(-)Deprenyl (selegiline), a catecholaminergic activity enhancer (CAE) substance acting in the brain". Pharmacol Toxicol. 82 (2): 57–66. doi:10.1111/j.1600-0773.1998.tb01399.x. PMID 9498233.
- ^ a b c d e f g h Miklya I (March 13, 2014). "The History of Selegiline/(-)-Deprenyl the First Selective Inhibitor of B-Type Monoamine Oxidase and The First Synthetic Catecholaminergic Activity Enhancer Substance". International Network for the History of Neuropsychopharmacology. Archived from the original on February 7, 2016. Retrieved January 7, 2016.
- ^ a b c d e f Gaszner P, Miklya I (January 2006). "Major depression and the synthetic enhancer substances, (-)-deprenyl and R-(-)-1-(benzofuran-2-yl)-2-propylaminopentane". Prog Neuropsychopharmacol Biol Psychiatry. 30 (1): 5–14. doi:10.1016/j.pnpbp.2005.06.004. PMID 16023777.
- ^ a b c d e f g h i j k Harsing LG, Timar J, Miklya I (August 2023). "Striking Neurochemical and Behavioral Differences in the Mode of Action of Selegiline and Rasagiline". Int J Mol Sci. 24 (17): 13334. doi:10.3390/ijms241713334. PMC 10487936. PMID 37686140.
- ^ a b c Berry MD (January 2007). "The potential of trace amines and their receptors for treating neurological and psychiatric diseases". Rev Recent Clin Trials. 2 (1): 3–19. doi:10.2174/157488707779318107. PMID 18473983.
In addition, the compounds previously described by Knoll and colleagues [33, 34], along with a series of trace amine derivatives synthesized by Ling et al. [35] are potential TAAR ligands. Although neither of these classes of compound appear to have been examined for efficacy at TAAR, their strong structural similarity to trace amines suggests that such studies are warranted.
- ^ a b c d e f g h i j k l m Gerlach M, Reichmann H, Riederer P (2012). "A critical review of evidence for preclinical differences between rasagiline and selegiline". Basal Ganglia. 2 (4): S9–S15. doi:10.1016/j.baga.2012.04.032.
At the preclinical level, there are some differences between rasagiline and selegiline (Table 1). Rasagiline is about 10 times more potent in the inhibition of MAO-B than selegiline in ex vivo studies. This higher potency of rasagiline is corrected in the clinic with dose adjustments (approved daily dose 1 and 5–10 mg for rasagiline and selegiline, respectively). Indeed, both agents lead to practically 100% inhibition of MAO-B in brain and platelets under therapeutic conditions [52–55].
- ^ a b c d e f Rothman RB, Baumann MH (October 2003). "Monoamine transporters and psychostimulant drugs". Eur J Pharmacol. 479 (1–3): 23–40. doi:10.1016/j.ejphar.2003.08.054. PMID 14612135.
- ^ a b c d e f g Kraemer T, Maurer HH (April 2002). "Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives". Ther Drug Monit. 24 (2): 277–289. doi:10.1097/00007691-200204000-00009. PMID 11897973.
- ^ a b c d Parnham MJ (1993). "The History of l-Deprenyl". Inhibitors of Monoamine Oxidase B: Pharmacology and Clinical Use in Neurodegenerative Disorders. Milestones in Drug Therapy. Basel: Birkhäuser Basel. pp. 237–251. doi:10.1007/978-3-0348-6348-3_12. ISBN 978-3-0348-6349-0.
- ^ a b c d e f g h i j Tábi T, Vécsei L, Youdim MB, Riederer P, Szökő É (May 2020). "Selegiline: a molecule with innovative potential". J Neural Transm (Vienna). 127 (5): 831–842. doi:10.1007/s00702-019-02082-0. PMC 7242272. PMID 31562557.
- ^ a b c d Seaman J, Landry JT (2011). Mylan: 50 Years of Unconventional Success: Making Quality Medicine Affordable and Accessible. University Press of New England. p. 50. ISBN 978-1-61168-269-4.
- ^ a b c Schneider LS, Tariot PN, Goldstein B (December 1994). "Therapy with l-deprenyl (selegiline) and relation to abuse liability". Clin Pharmacol Ther. 56 (6 Pt 2): 750–756. doi:10.1038/clpt.1994.205. PMID 7995017.
Some studies in patients with PD suggest that l-deprenyl might have relatively nonspecific ameliorative effects on mood and arousal.32,33 Indeed, the original reports in mixed psychiatric populations suggested a "psychic energizing" effect of the drug.34 Conceivably, such effects of l-deprenyl might be mediated through an increase in endogenous β-phenethylamine (β-PEA) consequent to MAO-B inhibition. β-PEA is a recognized psychomotor stimulant drug and, as higher levels develop in the presence of l-deprenyl, significant effects on mood might be anticipated (e.g., Greenshaw et al.35 and Timar and Knoll36).
- ^ a b c d Schifano F, Catalani V, Sharif S, Napoletano F, Corkery JM, Arillotta D, Fergus S, Vento A, Guirguis A (April 2022). "Benefits and Harms of 'Smart Drugs' (Nootropics) in Healthy Individuals". Drugs. 82 (6): 633–647. doi:10.1007/s40265-022-01701-7. PMID 35366192.
- ^ Blazer DG, Yaffe K, Liverman CT (July 21, 2015). Risk and Protective Factors and Interventions: General Cognitive Aging Interventions and Next Steps. National Academies Press (US). Retrieved July 5, 2024.
Other putative nootropics (e.g., selegiline, phosphadiylserine, atomoxetine, bupropion) have not been shown to have positive benefits on cognition in humans.
- ^ a b Brown RP, Gerbarg PL (2008). Muskin PR (ed.). "Integrative Psychopharmacology: A Practical Approach to Herbs and Nutrients in Psychiatry". Review of Psychiatry. Complementary and Alternative Medicine and Psychiatry. 19 (1). American Psychiatric Publishing: 1–66 (39). ISBN 978-1-58562-827-8. Retrieved July 5, 2024.
Selegiline (Eldepryl for humans [Somerset Pharmaceuticals, Tampa, FL] and Anipryl [Pfizer, New York, NY] for dogs) has been used as a treatment for Parkinson's disease. A powerful antioxidant in the brain, it has mild, amphetamine-like stimulant properties (Meeker and Reynolds 1990). Several human studies show slight improvements in cognitive function in early Alzheimer's disease (Mangoni et al. 1991). Anipryl has recently been approved for treatment of canine dementia. On the basis of one meager preliminary rat study, the alternative consumer literature often promotes selegiline as an antiaging drug. However, an antiaging effect has not been demonstrated in humans, and rat studies do not indicate what dosage might be adequate, if indeed such an effect exists.
- ^ a b c d e f g h i Finberg JP (April 2019). "Inhibitors of MAO-B and COMT: their effects on brain dopamine levels and uses in Parkinson's disease". Journal of Neural Transmission. 126 (4): 433–448. doi:10.1007/s00702-018-1952-7. PMID 30386930.
Rasagiline had an ED50 for inhibition of MAO-A in rat brain of 6.5 mg/kg and 0.1 mg/kg for MAO-B, giving a ratio of selectivity MAO-A: MAO-B of 65, while for selegiline the figures were 34 and 0.35 mg/kg, respectively, giving a ratio of 98 (measurements made 2 h after a single oral dose of the compounds). The selectivity of these drugs for MAO-B is therefore not absolute, as will be discussed in the following.
- ^ a b c d "Drugs@FDA: FDA-Approved Drugs". accessdata.fda.gov. Retrieved July 1, 2024.
- ^ a b c d e f g h i j k l Asnis GM, Henderson MA (2014). "EMSAM (deprenyl patch): how a promising antidepressant was underutilized". Neuropsychiatr Dis Treat. 10: 1911–1923. doi:10.2147/NDT.S59107. PMC 4200016. PMID 25336957.
- ^ Riederer P, Lachenmayer L, Laux G (August 2004). "Clinical applications of MAO-inhibitors". Current Medicinal Chemistry. 11 (15): 2033–2043. doi:10.2174/0929867043364775 (inactive April 2, 2024). PMID 15279566.
{{cite journal}}: CS1 maint: DOI inactive as of April 2024 (link) - ^ a b c d "Selegiline Hydrochloride Monograph for Professionals". Drugs.com. Retrieved February 23, 2018.
- ^ a b Ives NJ, Stowe RL, Marro J, Counsell C, Macleod A, Clarke CE, Gray R, Wheatley K (September 2004). "Monoamine oxidase type B inhibitors in early Parkinson's disease: meta-analysis of 17 randomised trials involving 3525 patients". BMJ. 329 (7466): 593. doi:10.1136/bmj.38184.606169.AE. PMC 516655. PMID 15310558.
- ^ Riederer P, Lachenmayer L (November 2003). "Selegiline's neuroprotective capacity revisited". Journal of Neural Transmission. 110 (11): 1273–1278. doi:10.1007/s00702-003-0083-x. PMID 14628191. S2CID 20232921.
- ^ Frisina PG, Tenenbaum HR, Borod JC, Foldi NS (May 2008). "The effects of antidepressants in Parkinson's disease: a meta-analysis". Int J Neurosci. 118 (5): 667–682. doi:10.1080/00207450701239418. PMID 18446583.
- ^ Tsuboi T, Satake Y, Hiraga K, Yokoi K, Hattori M, Suzuki M, Hara K, Ramirez-Zamora A, Okun MS, Katsuno M (June 2022). "Effects of MAO-B inhibitors on non-motor symptoms and quality of life in Parkinson's disease: A systematic review". npj Parkinsons Dis. 8 (1): 75. doi:10.1038/s41531-022-00339-2. PMC 9192747. PMID 35697709.
- ^ a b Binde CD, Tvete IF, Gåsemyr J, Natvig B, Klemp M (September 2018). "A multiple treatment comparison meta-analysis of monoamine oxidase type B inhibitors for Parkinson's disease". Br J Clin Pharmacol. 84 (9): 1917–1927. doi:10.1111/bcp.13651. PMC 6089809. PMID 29847694.
- ^ Hengartner MP, Jakobsen JC, Sørensen A, Plöderl M (2020). "Efficacy of new-generation antidepressants assessed with the Montgomery-Asberg Depression Rating Scale, the gold standard clinician rating scale: A meta-analysis of randomised placebo-controlled trials". PLOS ONE. 15 (2): e0229381. Bibcode:2020PLoSO..1529381H. doi:10.1371/journal.pone.0229381. PMC 7043778. PMID 32101579.
- ^ Pae CU, Patkar AA, Jang S, Portland KB, Jung S, Nelson JC (August 2014). "Efficacy and safety of selegiline transdermal system (STS) for the atypical subtype of major depressive disorder: pooled analysis of 5 short-term, placebo-controlled trials". CNS Spectr. 19 (4): 324–329. doi:10.1017/S1092852913000655. PMID 24168807.
- ^ a b c d Clayton AH, Campbell BJ, Favit A, Yang Y, Moonsammy G, Piontek CM, Amsterdam JD (December 2007). "Symptoms of sexual dysfunction in patients treated for major depressive disorder: a meta-analysis comparing selegiline transdermal system and placebo using a patient-rated scale". J Clin Psychiatry. 68 (12): 1860–1866. doi:10.4088/jcp.v68n1205. PMID 18162016.
- ^ Winter J, Curtis K, Hu B, Clayton AH (July 2022). "Sexual dysfunction with major depressive disorder and antidepressant treatments: impact, assessment, and management". Expert Opin Drug Saf. 21 (7): 913–930. doi:10.1080/14740338.2022.2049753. PMID 35255754.
SSRIs are associated with 70% treatment-emergent sexual dysfunction (TESD), SNRIs and tricyclics have rates of TESD of 40–45%, and antidepressant medications without SRI effects or with additional unique mechanisms of action have rates similar to placebo (<10%).
- ^ a b c d e f g h i Hoffman GR, Olson MG, Schoffstall AM, Estévez RF, Van den Eynde V, Gillman PK, Stabio ME (December 2023). "Classics in Chemical Neuroscience: Selegiline, Isocarboxazid, Phenelzine, and Tranylcypromine". ACS Chem Neurosci. 14 (23): 4064–4075. doi:10.1021/acschemneuro.3c00591. PMID 37966854.
- ^ a b c d e f g h i j Knoll J (1983). "Deprenyl (selegiline): the history of its development and pharmacological action". Acta Neurol Scand Suppl. 95: 57–80. doi:10.1111/j.1600-0404.1983.tb01517.x. PMID 6428148.
- ^ a b c d e f Alborghetti M, Nicoletti F (2019). "Different Generations of Type-B Monoamine Oxidase Inhibitors in Parkinson's Disease: From Bench to Bedside". Curr Neuropharmacol. 17 (9): 861–873. doi:10.2174/1570159X16666180830100754. PMC 7052841. PMID 30160213.
- ^ Friedman RA, Leon AC (June 2007). "Expanding the black box - depression, antidepressants, and the risk of suicide". The New England Journal of Medicine. 356 (23): 2343–2346. doi:10.1056/NEJMp078015. PMID 17485726.
- ^ Olanow CW, Myllylä VV, Sotaniemi KA, Larsen JP, Pålhagen S, Przuntek H, Heinonen EH, Kilkku O, Lammintausta R, Mäki-Ikola O, Rinne UK (September 1998). "Effect of selegiline on mortality in patients with Parkinson's disease: a meta-analysis". Neurology. 51 (3): 825–830. doi:10.1212/wnl.51.3.825. PMID 9748034.
- ^ Aaltonen H, Kilkku O, Heinonen E, Mäki-Ikola O (December 1998). "Effect of adding selegiline to levodopa in early, mild Parkinson's disease. Evidence is insufficient to show that combined treatment increases mortality". BMJ. 317 (7172): 1586–1587. doi:10.1136/bmj.317.7172.1586. PMC 1114394. PMID 9890764.
- ^ a b Roy MA, Doiron M, Talon-Croteau J, Dupré N, Simard M (July 2018). "Effects of Antiparkinson Medication on Cognition in Parkinson's Disease: A Systematic Review". Can J Neurol Sci. 45 (4): 375–404. doi:10.1017/cjn.2018.21. PMID 29747716.
- ^ a b Vitale C, Amboni M, Erro R, Picillo M, Pellecchia MT, Barone P, Trojano L, Santangelo G (June 2019). "Parkinson's disease management and impulse control disorders: current state and future perspectives". Expert Rev Neurother. 19 (6): 495–508. doi:10.1080/14737175.2019.1620603. PMID 31148487.
The association between ICD and the use of MAO and COMT inhibitors appears to be controversial. There are few reports implicating monoamine oxidase-B inhibitors (selegiline and rasagiline) in ICDs, although some of these patients were already taking other antiparkinsonian medication [82–86]. In all of these cases, the addition of MAO inhibitors caused the onset or recurrence of ICDs, with complete remission of behavioral symptoms after suspension. Garcia-Ruiz et al., found an association between ICDs and concomitant treatment with rasagiline, with an OR of 2.2 [70]. These findings suggest that MAO inhibitors may enhance the already high risk of developing ICDs in some predisposed PD patients taking DRT, by increasing dopamine plasma levels, but further studies are warranted to confirm this hypothesis.
- ^ Solla P, Bortolato M, Cannas A, Mulas CS, Marrosu F (April 2015). "Paraphilias and paraphilic disorders in Parkinson's disease: A systematic review of the literature". Mov Disord. 30 (5): 604–613. doi:10.1002/mds.26157. PMC 4428164. PMID 25759330.
- ^ Hirao K, Kaneko Y, Hirose D, Fukasawa R, Shimizu S, Kanetaka H, Umahara T, Sakurai H, Hanyu H (September 2019). "Patient with Parkinson's disease presenting with impulse control disorders following treatment with selegiline". Int Psychogeriatr. 31 (9): 1375–1376. doi:10.1017/S1041610218001862. PMID 30520410.
- ^ Uitti RJ, Tanner CM, Rajput AH, Goetz CG, Klawans HL, Thiessen B (October 1989). "Hypersexuality with antiparkinsonian therapy". Clin Neuropharmacol. 12 (5): 375–383. doi:10.1097/00002826-198910000-00002. PMID 2575449.
- ^ Riley DE (2002). "Reversible transvestic fetishism in a man with Parkinson's disease treated with selegiline". Clin Neuropharmacol. 25 (4): 234–237. doi:10.1097/00002826-200207000-00008. PMID 12151912.
- ^ Shapiro MA, Chang YL, Munson SK, Okun MS, Fernandez HH (September 2006). "Hypersexuality and paraphilia induced by selegiline in Parkinson's disease: report of 2 cases". Parkinsonism Relat Disord. 12 (6): 392–395. doi:10.1016/j.parkreldis.2006.01.010. PMID 16730214.
- ^ Howell M, Avidan AY, Foldvary-Schaefer N, Malkani RG, During EH, Roland JP, McCarter SJ, Zak RS, Carandang G, Kazmi U, Ramar K (April 2023). "Management of REM sleep behavior disorder: an American Academy of Sleep Medicine clinical practice guideline". J Clin Sleep Med. 19 (4): 759–768. doi:10.5664/jcsm.10424. PMC 10071384. PMID 36515157.
- ^ Hoque R, Chesson AL (February 2010). "Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis". J Clin Sleep Med. 6 (1): 79–83. doi:10.5664/jcsm.27716. PMC 2823282. PMID 20191944.
- ^ Louden MB, Morehead MA, Schmidt HS (1995). "Activation by selegiline (Eldepryle) of REM sleep behavior disorder in parkinsonism". W V Med J. 91 (3): 101. PMID 7747490.
- ^ a b Winger GD, Yasar S, Negus SS, Goldberg SR (December 1994). "Intravenous self-administration studies with l-deprenyl (selegiline) in monkeys". Clin Pharmacol Ther. 56 (6 Pt 2): 774–780. doi:10.1038/clpt.1994.208. hdl:2027.42/110034. PMID 7995020.
l-Deprenyl and its stereoisomer d-deprenyl did not maintain intravenous self-administration behavior in rhesus monkeys. In contrast, l-methamphetamine, the major metabolite of l-deprenyl, as well as the baseline drug, cocaine, maintained high rates of intravenous self-administration behavior. Treatment with l-deprenyl doses up to 1.0 mg/kg before self-administration sessions failed to alter self-administration of either cocaine or l-methamphetamine. Thus l-deprenyl did not appear to have cocaine- or methamphetamine-like reinforcing properties in monkeys and was ineffective in altering established patterns of psychomotor-stimulant self-administration behavior. These results support clinical findings that despite long-term use of l-deprenyl for the treatment of Parkinson's disease by large numbers of patients, no instances of abuse have been documented.
- ^ a b Yasar S, Gaál J, Panlilio LV, Justinova Z, Molnár SV, Redhi GH, Schindler CW (January 2006). "A comparison of drug-seeking behavior maintained by D-amphetamine, L-deprenyl (selegiline), and D-deprenyl under a second-order schedule in squirrel monkeys". Psychopharmacology (Berl). 183 (4): 413–421. doi:10.1007/s00213-005-0200-7. PMC 1360227. PMID 16292593.
D-Amphetamine maintained high rates of drug-seeking behavior on the second-order schedule. D-Deprenyl maintained high rates of drug-seeking behavior similar to D-amphetamine. L-Deprenyl maintained lower rates of responding that were not significantly above saline substitution levels. Pretreatment with L-deprenyl failed to alter drug-seeking behavior maintained by D-amphetamine. These results indicate that D-deprenyl, but not L-deprenyl, may have abuse potential.
- ^ McKean AJ, Leung JG, Dare FY, Sola CL, Schak KM (2015). "The Perils of Illegitimate Online Pharmacies: Substance-Induced Panic Attacks and Mood Instability Associated With Selegiline and Phenylethylamine". Psychosomatics. 56 (5): 583–587. doi:10.1016/j.psym.2015.05.003. PMID 26198572.
- ^ Monteith S, Glenn T, Bauer R, Conell J, Bauer M (March 2016). "Availability of prescription drugs for bipolar disorder at online pharmacies". J Affect Disord. 193: 59–65. doi:10.1016/j.jad.2015.12.043. PMID 26766033.
Other examples of serious adverse reactions in patients who took self-prescribed medications from the Internet include lithium toxicity from a dietary supplement (Pauzé and Brooks, 2007), severe hypotension from high-dose niacin by a patient with schizophrenia (Mularski et al., 2006), and panic attacks from selegiline and phenylethylamine by a patient with depression (McKean et al., 2015).
- ^ Kuhn W, Müller T (1996). "The clinical potential of Deprenyl in neurologic and psychiatric disorders". Deprenyl — Past and Future. Vol. 48. pp. 85–93. doi:10.1007/978-3-7091-7494-4_8. ISBN 978-3-211-82891-5. PMID 8988464.
{{cite book}}:|journal=ignored (help) - ^ a b c Heinonen EH, Myllylä V (July 1998). "Safety of selegiline (deprenyl) in the treatment of Parkinson's disease". Drug Safety. 19 (1): 11–22. doi:10.2165/00002018-199819010-00002. PMID 9673855. S2CID 9632549.
- ^ Csoti I, Storch A, Müller W, Jost WH (December 1, 2012). "Drug interactions with selegiline versus rasagiline". Basal Ganglia. Monoamine oxidase B Inhibitors. 2 (4, Supplement): S27–S31. doi:10.1016/j.baga.2012.06.003. ISSN 2210-5336.
- ^ Gillman PK (October 2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210. PMID 16051647.
- ^ Jessen L, Kovalick LJ, Azzaro AJ (April 2008). "The selegiline transdermal system (emsam): a therapeutic option for the treatment of major depressive disorder". P & T. 33 (4): 212–246. PMC 2730099. PMID 19750165.
- ^ a b c d Laine K, Anttila M, Nyman L, Wahlberg A, Bertilsson L (May 2001). "CYP2C19 polymorphism is not important for the in vivo metabolism of selegiline". Eur J Clin Pharmacol. 57 (2): 137–142. doi:10.1007/s002280100289. PMID 11417445.
Selegiline is metabolised to two known metabolites, desmethylselegiline and l-methamphetamine (Fig. 1) [7]. The oral bioavailability of selegiline is less than 10% due to extensive first-pass metabolism [8] and the concentrations of these two metabolites are 10- to 20-fold higher in serum compared with the parent compound [6, 9].
- ^ a b Kivistö KT, Wang JS, Backman JT, Nyman L, Taavitsainen P, Anttila M, Neuvonen PJ (April 2001). "Selegiline pharmacokinetics are unaffected by the CYP3A4 inhibitor itraconazole". Eur J Clin Pharmacol. 57 (1): 37–42. doi:10.1007/s002280100278. PMID 11372588.
- ^ a b "Table of Substrates, Inhibitors and Inducers". U.S. Food and Drug Administration. June 5, 2023. Retrieved July 5, 2024.
- ^ Naoi M, Maruyama W, Shamoto-Nagai M (September 2022). "Neuroprotective Function of Rasagiline and Selegiline, Inhibitors of Type B Monoamine Oxidase, and Role of Monoamine Oxidases in Synucleinopathies". Int J Mol Sci. 23 (19): 11059. doi:10.3390/ijms231911059. PMC 9570229. PMID 36232361.
Oral administered selegiline is rapidly metabolized in the liver by CYP2B6 and a lesser extent by CYP2A6 and CYP3A4 into L-methamphetamine, which is further metabolized to L-amphetamine and desmethylselegiline [59].
- ^ a b c Klietz M, Greten S, Wegner F, Höglinger GU (June 2019). "Safety and Tolerability of Pharmacotherapies for Parkinson's Disease in Geriatric Patients". Drugs & Aging. 36 (6): 511–530. doi:10.1007/s40266-019-00654-z. PMID 30937878.
Another interaction exists between selegiline and female sex hormones. According to Laine et al., oral contraceptives (estradiol/levonorgestrel) markedly increase the oral bioavailability of selegiline [184]. For postmenopausal hormone replacement therapy, this interaction could not be confrmed [185]. Therefore, it is not relevant for geriatric patients with PD.
- ^ a b c d Laine K, Anttila M, Helminen A, Karnani H, Huupponen R (March 1999). "Dose linearity study of selegiline pharmacokinetics after oral administration: evidence for strong drug interaction with female sex steroids". Br J Clin Pharmacol. 47 (3): 249–254. doi:10.1046/j.1365-2125.1999.00891.x. PMC 2014223. PMID 10215747.
- ^ a b Palovaara S, Anttila M, Nyman L, Laine K (July 2002). "Effect of concomitant hormone replacement therapy containing estradiol and levonorgestrel on the pharmacokinetics of selegiline". European Journal of Clinical Pharmacology. 58 (4): 259–263. doi:10.1007/s00228-002-0469-y. PMID 12136372.
- ^ a b c d e Zanger UM, Klein K (2013). "Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): advances on polymorphisms, mechanisms, and clinical relevance". Front Genet. 4: 24. doi:10.3389/fgene.2013.00024. PMC 3588594. PMID 23467454.
- ^ a b c Hedrich WD, Hassan HE, Wang H (September 2016). "Insights into CYP2B6-mediated drug-drug interactions". Acta Pharm Sin B. 6 (5): 413–425. doi:10.1016/j.apsb.2016.07.016. PMC 5045548. PMID 27709010.
Selegiline is frequently used in the treatment of Parkinson's disease. Sridar et al.178 have shown selegiline to be a strong inhibitor of CYP2B6-mediated metabolism of bupropion in vitro, increasing the Km of bupropion from 10 to 92 mmol/L and decreasing the kcat by approximately 50%178. This strong inhibition of CYP2B6 by selegiline highlights a serious potential of DDI for combination therapies involving bupropion.
- ^ a b c d Sridar C, Kenaan C, Hollenberg PF (December 2012). "Inhibition of bupropion metabolism by selegiline: mechanism-based inactivation of human CYP2B6 and characterization of glutathione and peptide adducts". Drug Metab Dispos. 40 (12): 2256–2266. doi:10.1124/dmd.112.046979. PMC 3500550. PMID 22936314.
- ^ a b Nirogi R, Palacharla RC, Mohammed AR, Manoharan A, Ponnamaneni RK, Bhyrapuneni G (March 2015). "Evaluation of metabolism dependent inhibition of CYP2B6 mediated bupropion hydroxylation in human liver microsomes by monoamine oxidase inhibitors and prediction of potential as perpetrators of drug interaction". Chem Biol Interact. 230: 9–20. Bibcode:2015CBI...230....9N. doi:10.1016/j.cbi.2015.01.028. PMID 25656918.
- ^ Ritter JL, Alexander B (March 1997). "Retrospective study of selegiline-antidepressant drug interactions and a review of the literature". Ann Clin Psychiatry. 9 (1): 7–13. doi:10.1023/a:1026222106851. PMID 9167831.
- ^ Tanner JA, Tyndale RF (December 2017). "Variation in CYP2A6 Activity and Personalized Medicine". J Pers Med. 7 (4): 18. doi:10.3390/jpm7040018. PMC 5748630. PMID 29194389.
Both mechanism-based and competitive inhibitors act on the CYP2A6 enzyme. 8-Methoxypsoralen, also known as methoxsalen, and selegiline are examples of mechanism-based inhibitors (MBIs) of CYP2A6 [8,64]. Methoxsalen is used to treat skin conditions, including psoriasis and eczema [65,66]. Selegiline is a monoamine oxidase (MAO) inhibitor that is used in the early treatment of Parkinson's disease [67,68]. As MBIs, methoxsalen and selegiline are metabolically activated by CYP2A6, and their products subsequently irreversibly bind to and inhibit CYP2A6 [69]. Another MAO inhibitor, tranylcypromine, acts as a competitive inhibitor of CYP2A6 in vitro with coumarin used as a substrate of CYP2A6 [70].
- ^ a b c d e Siu EC, Tyndale RF (March 2008). "Selegiline is a mechanism-based inactivator of CYP2A6 inhibiting nicotine metabolism in humans and mice". J Pharmacol Exp Ther. 324 (3): 992–9. doi:10.1124/jpet.107.133900. PMID 18065502.
Selegiline also seemed to be able to inhibit its own metabolism in vivo (Laine et al., 2000), possibly via inhibition of CYP2A6. [...] Selegiline is metabolized to desmethylselegiline and L-methamphetamine, both of which can be further metabolized to L-amphetamine as well as other minor metabolites (Fig. 1) (Shin, 1997; Valoti et al., 2000); despite this, there is no evidence indicating that selegiline is addictive (Schneider et al., 1994). In humans, chronic treatment with selegiline reduces the metabolism of selegiline and its metabolites, suggesting that selegiline or its metabolites may inhibit or down-regulate its own metabolic enzymes (Laine et al., 2000). Selegiline belongs to the acetylene group of compounds that contain a carboncarbon triple bond, which are known to be potent mechanismbased inhibitors (Correia and Ortiz de Montellano, 2005).
- ^ a b c Laine K, Anttila M, Huupponen R, Mäki-Ikola O, Heinonen E (2000). "Multiple-dose pharmacokinetics of selegiline and desmethylselegiline suggest saturable tissue binding". Clin Neuropharmacol. 23 (1): 22–27. doi:10.1097/00002826-200001000-00005. PMID 10682227.
- ^ a b Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J (2008). "Pharmacotherapy of methamphetamine addiction: an update". Subst Abus. 29 (3): 31–49. doi:10.1080/08897070802218554. PMC 2597382. PMID 19042205.
- ^ a b Newton TF, De La Garza R, Fong T, Chiang N, Holmes TH, Bloch DA, Anderson A, Elkashef A (December 2005). "A comprehensive assessment of the safety of intravenous methamphetamine administration during treatment with selegiline". Pharmacol Biochem Behav. 82 (4): 704–711. doi:10.1016/j.pbb.2005.11.012. PMID 16413604.
- ^ a b Finberg JP (August 2014). "Update on the pharmacology of selective inhibitors of MAO-A and MAO-B: focus on modulation of CNS monoamine neurotransmitter release". Pharmacol Ther. 143 (2): 133–152. doi:10.1016/j.pharmthera.2014.02.010. PMID 24607445.
- ^ a b c Houtsmuller EJ, Notes LD, Newton T, van Sluis N, Chiang N, Elkashef A, Bigelow GE (February 2004). "Transdermal selegiline and intravenous cocaine: safety and interactions". Psychopharmacology (Berl). 172 (1): 31–40. doi:10.1007/s00213-003-1616-6. PMID 14605792.
- ^ a b Bartzokis G, Beckson M, Newton T, Mandelkern M, Mintz J, Foster JA, Ling W, Bridge TP (June 1999). "Selegiline effects on cocaine-induced changes in medial temporal lobe metabolism and subjective ratings of euphoria". Neuropsychopharmacology. 20 (6): 582–590. doi:10.1016/S0893-133X(98)00092-X. PMID 10327427.
- ^ a b Haberny KA, Walsh SL, Ginn DH, Wilkins JN, Garner JE, Setoda D, Bigelow GE (July 1995). "Absence of acute cocaine interactions with the MAO-B inhibitor selegiline". Drug Alcohol Depend. 39 (1): 55–62. doi:10.1016/0376-8716(95)01137-n. PMID 7587975.
- ^ a b Harris DS, Everhart T, Jacob P, Lin E, Mendelson JE, Jones RT (August 2009). "A phase 1 trial of pharmacologic interactions between transdermal selegiline and a 4-hour cocaine infusion". BMC Clin Pharmacol. 9: 13. doi:10.1186/1472-6904-9-13. PMC 2731040. PMID 19646280.
- ^ a b Newton TF, Kalechstein A, Beckson M, Bartzokis G, Bridge TP, Ling W (October 1999). "Effects of selegiline pretreatment on response to experimental cocaine administration". Psychiatry Res. 87 (2–3): 101–106. doi:10.1016/s0165-1781(99)00058-x. PMID 10579543.
- ^ Knoll J (September 1992). "Pharmacological basis of the therapeutic effect of (-)deprenyl in age-related neurological diseases". Med Res Rev. 12 (5): 505–524. doi:10.1002/med.2610120504. PMID 1513186.
- ^ a b c d e f g Knoll J (May 1992). "The pharmacological profile of (-)deprenyl (selegiline) and its relevance for humans: a personal view". Pharmacology & Toxicology. 70 (5 Pt 1): 317–321. doi:10.1111/j.1600-0773.1992.tb00480.x. PMID 1608919.
- ^ a b Birks J, Flicker L (2003). "Selegiline for Alzheimer's disease". Cochrane Database Syst Rev (1): CD000442. doi:10.1002/14651858.CD000442. PMID 12535396.
MAO-B accounts for some 80% of human brain MAO, and uses dopamine and phenylethylamine as substrates. It is selectively inhibited by selegiline. Selegiline has been shown to inhibit the uptake of dopamine in the presynaptic nerve endings thus increasing its synthesis. Beside potentiating endogenous dopaminergic functions of the nigrostriatal (motor) neuronal systems affected in Parkinson's disease, selegiline may also influence mesolimbic (cognitive) neuronal systems that loop through frontocortical pathways.
- ^ Magyar K, Pálfi M, Tábi T, Kalász H, Szende B, Szöko E (August 2004). "Pharmacological aspects of (-)-deprenyl". Curr Med Chem. 11 (15): 2017–31. doi:10.2174/0929867043364793. PMID 15279565.
(-)-Deprenyl and probably all of the MAO-B inhibitors potentiate DA effect not only in the nigrostriatal pathway, important in motor control, but the mesolimbic/mesocortical pathways (limbic system) as well, which are involved in emotion and drug induced reward system. The dopaminergic system in the tuberohypophyseal pathway and the chemoreceptor trigger zone is also potentiated. These effects may well explain adverse events, observed in clinical studies. [...] (-)-Deprenyl potentiates DA effects in the nigrostriatal pathway, which improves movement disorder [26], but also enhances dopaminergic activity in the mesolimbic/mesocortical pathways, the tuberohypophyseal bundle and the chemosensitive trigger zone. These effects may explain the adverse events observed during prolonged administration of (-)-deprenyl with levodopa in human therapy.
- ^ Magyar K, Szende B (January 2004). "(-)-Deprenyl, a selective MAO-B inhibitor, with apoptotic and anti-apoptotic properties". Neurotoxicology. 25 (1–2): 233–242. doi:10.1016/S0161-813X(03)00102-5. PMID 14697898.
(-)-Deprenyl and probably all of the MAO-B inhibitors potentiate DA effect not only on the nigrostriatal pathway, important in motor control, but the mesolimbic/mesocortical pathways (limbic system) as well, which are involved in emotion- and drug-induced reward system. The dopaminergic system in the tuberohypophyseal pathway and the chemoreceptor trigger zone is also potentiated. These effects may well explain adverse events, observed in clinical studies. [...] When (-)-deprenyl is chronically administered with levodopa in a dose needed to induce MAO-B inhibition, it potentiates DA effects not only in the nigrostriatal pathway (which is the aim in Parkinson's disease), but also enhance DA activity in the mesolimbic/mesocortical pathways. Furthermore, the tuberohypophyseal bundle and the chemosensitive trigger zone are also involved in DA potentiation. These effects may explain the adverse events observed during prolonged administration of (-)-deprenyl with levodopa in human therapy.
- ^ a b Naoi M, Maruyama W, Shamoto-Nagai M, Riederer P (June 2024). "Toxic interactions between dopamine, α-synuclein, monoamine oxidase, and genes in mitochondria of Parkinson's disease". J Neural Transm (Vienna). 131 (6): 639–661. doi:10.1007/s00702-023-02730-6. PMID 38196001.
- ^ Oreland L, Johansson F, Ekstedt J (1983). "Dose regimen of deprenyl (selegiline) and platelet MAO activities". Acta Neurologica Scandinavica. Supplementum. 95: 87–89. doi:10.1111/j.1600-0404.1983.tb01519.x. PMID 6428150.
- ^ Teychenne PF, Parker S (1989). "Double-blind, crossover placebo controlled trial of selegiline in Parkinson's disease--an interim analysis". Acta Neurologica Scandinavica. Supplementum. 126: 119–125. doi:10.1111/j.1600-0404.1989.tb01791.x. PMID 2515717.
- ^ Finberg JP, Gillman K (2011). Selective inhibitors of monoamine oxidase type B and the "cheese effect". International Review of Neurobiology. Vol. 100. pp. 169–190. doi:10.1016/B978-0-12-386467-3.00009-1. ISBN 978-0-12-386467-3. PMID 21971008.
As these propargyl inhibitors efficiently pass the blood–brain barrier, it may be assumed that the extent of MAO-B inhibition for a given dose in the brain is very close to that in the periphery, and animal experiments show this to be true (see Waldmeieret al., 1981).
- ^ a b Riederer P, Youdim MB (May 1986). "Monoamine oxidase activity and monoamine metabolism in brains of parkinsonian patients treated with l-deprenyl". Journal of Neurochemistry. 46 (5): 1359–1365. doi:10.1111/j.1471-4159.1986.tb01747.x. PMID 2420928.
In the brains, where MA0 activity toward DA has been shown to be almost completely inhibited as a result of 1-deprenyl therapy (Table 3), DA levels in caudate nucleus, putamen, globus pallidus, and substantia nigra rose to about 40-50% of control values (Fig. 2). Although brain 5-HT levels in non-1-deprenyl-treated Parkinsonian patients were lower than corresponding controls for the same regions (30-50% of control values), l-deprenyl therapy hardly affected its concentration (Fig. 3). In contrast, brain 5-HIAA concentrations are not altered in Parkinsonian patients (Fig. 3). However, it is apparent that I-deprenyl may lower 5-HIAA. This was found to be significant only in nucleus amygdala (p < 0.05). [...] Postmortem studies of human brain have already shown that after 1-deprenyl administration in Parkinsonian subjects, PEA concentrations are substantially increased in the striatum (Reynolds et al., 1978; Riederer et al., 1986) by > 1,300%. The fact that l-deprenyl also induces significant increases of DA in the caudate nucleus, globus pallidus, putamen, and substantia nigra, without a marked change in either 5-HT or 5- HIAA, would be indicative of the importance of MA0 B for DA deamination in human brain. This conclusion is supported by the near-total inhibition of DA deamination as measured in ex vivo experiments, using homogenates of brain regions from 1- deprenyl-treated Parkinson patients.
- ^ a b Fowler JS, Volkow ND, Logan J, Wang GJ, MacGregor RR, Schyler D, Wolf AP, Pappas N, Alexoff D, Shea C (October 1994). "Slow recovery of human brain MAO B after L-deprenyl (Selegeline) withdrawal". Synapse. 18 (2): 86–93. doi:10.1002/syn.890180203. PMID 7839316.
- ^ a b c Riederer P, Jellinger K, Seemann D (1984). "Monoamine Oxidase and Parkinsonism". In Tipton KF, Dostert P, Benedetti MS (eds.). Monoamine Oxidase and Disease: Prospects for Therapy with Reversible Inhibitors. Academic Press rapid manuscript reproduction. Academic Press. pp. 404–415. ISBN 978-0-12-691660-7. Retrieved July 5, 2024.
In addition, Table 1 gives evidence for a major role of DA and, particularly, of PEA in the therapeutic action of (-) deprenyl (36, 37). [...] TABLE 1: Biogenic Amines in Parkinson's Disease Treated with (-) Deprenyl. Amine: Serotonin: Striatum % increase: 5 (9). Limbic Areas % increase: 0 (3). Amine: Dopamine: Striatum % increase: 23 (4). Limbic Areas % increase: 41 (4). Amine: PEA: Striatum % increase: 1192 (10). Limbic Areas % increase: 3440 (6). dose of (-) deprenyl: 10 mg/day. duration of treatment: 3 to 8 days. ( ) number of determinations.
- ^ Riederer P, Youdim MB, Rausch WD, Birkmayer W, Jellinger K, Seemann D (1978). "On the mode of action of L-deprenyl in the human central nervous system". Journal of Neural Transmission. 43 (3–4): 217–226. doi:10.1007/BF01246958. PMID 745014.
- ^ Reynolds GP, Riederer P, Sandler M, Jellinger K, Seemann D (1978). "Amphetamine and 2-phenylethylamine in post-mortem Parkinsonian brain after (-)deprenyl administration". Journal of Neural Transmission. 43 (3–4): 271–277. doi:10.1007/BF01246964. PMID 745019.
- ^ a b c Fowler JS, Logan J, Volkow ND, Shumay E, McCall-Perez F, Jayne M, Wang GJ, Alexoff DL, Apelskog-Torres K, Hubbard B, Carter P, King P, Fahn S, Gilmor M, Telang F, Shea C, Xu Y, Muench L (February 2015). "Evidence that formulations of the selective MAO-B inhibitor, selegiline, which bypass first-pass metabolism, also inhibit MAO-A in the human brain". Neuropsychopharmacology. 40 (3): 650–657. doi:10.1038/npp.2014.214. PMC 4289953. PMID 25249059.
- ^ Birkmayer W, Riederer P, Youdim MB (1982). "(-)Deprenyl in the treatment of Parkinson's disease". Clin Neuropharmacol. 5 (2): 195–230. doi:10.1097/00002826-198205020-00004. PMID 6814755.
There is a correlation between these studies and the appearance of behavioral change in rats that is directly dependent on the inhibition of both MAO type A and type B by more than 85%; [...]
- ^ Elsworth JD, Glover V, Reynolds GP, Sandler M, Lees AJ, Phuapradit P, Shaw KM, Stern GM, Kumar P (April 1978). "Deprenyl administration in man: a selective monoamine oxidase B inhibitor without the 'cheese effect'". Psychopharmacology (Berl). 57 (1): 33–38. doi:10.1007/BF00426954. PMID 96466.
Urinary phenylethylamine output was monitored in the volunteers and a typical response is shown in Figure 1. Concentration was not altered by 1 mg (-)- deprenyl, but rose strikingly as the dose was increased, falling sharply after the drug was withdrawn. The 10- mg dose caused a 20 to 90-fold rise in phenylethylamine excretion in the volunteers compared with their control values.
- ^ a b c Janssen PA, Leysen JE, Megens AA, Awouters FH (September 1999). "Does phenylethylamine act as an endogenous amphetamine in some patients?". Int J Neuropsychopharmacol. 2 (3): 229–240. doi:10.1017/S1461145799001522. PMID 11281991.
The use of selegiline (Deprenyl) at a dose of 10 mg}d indirectly increases PEA concentrations by inhibition of MAO-B, whereas DA metabolism is not affected, at least not in the rat (Paterson et al., 1991). [...] Finally, although there is no consistent evidence of a PEA deficit in depression (Davis and Boulton, 1994), a sustained antidepressant effect of PEA replacement (oral PEA 10–60 mg}d, with 10 mg selegiline) has been reported in patients with major depressive episodes (Sabelli et al., 1996).
- ^ Riederer P, Laux G (March 2011). "MAO-inhibitors in Parkinson's Disease". Exp Neurobiol. 20 (1): 1–17. doi:10.5607/en.2011.20.1.1. PMC 3213739. PMID 22110357.
It is not far-fetched to assume, that PEA, which increases after selegiline treatment in brain tissue (Reynolds et al., 1978) and by this exerts dopamine release-promoting properties, contributes to dopamine's behavioural effects including improvement of motility (Foley, 2001; Gerlach et al., 2007; Riederer, 2009).
- ^ a b Yasar S, Justinova Z, Lee SH, Stefanski R, Goldberg SR, Tanda G (April 2006). "Metabolic transformation plays a primary role in the psychostimulant-like discriminative-stimulus effects of selegiline [(R)-(-)-deprenyl]". J Pharmacol Exp Ther. 317 (1): 387–394. doi:10.1124/jpet.105.096263. PMID 16352699.
- ^ a b Heinonen EH, Anttila MI, Karnani HL, Nyman LM, Vuorinen JA, Pyykkö KA, Lammintausta RA (July 1997). "Desmethylselegiline, a metabolite of selegiline, is an irreversible inhibitor of monoamine oxidase type B in humans". J Clin Pharmacol. 37 (7): 602–609. doi:10.1002/j.1552-4604.1997.tb04342.x. PMID 9243353.
- ^ a b c Nadeem MS, Hosawi SB, Murtaza BN, Kazmi I (2023). "Mechanism of action of anti-Parkinson's drugs". How Synthetic Drugs Work: Insights Into Molecular Pharmacology of Classic and New Pharmaceuticals. Elsevier. pp. 195–213. doi:10.1016/b978-0-323-99855-0.00009-9. ISBN 978-0-323-99855-0.
- ^ a b c Nam MH, Sa M, Ju YH, Park MG, Lee CJ (April 2022). "Revisiting the Role of Astrocytic MAOB in Parkinson's Disease". International Journal of Molecular Sciences. 23 (8): 4453. doi:10.3390/ijms23084453. PMC 9028367. PMID 35457272.
- ^ a b c Cho HU, Kim S, Sim J, Yang S, An H, Nam MH, Jang DP, Lee CJ (July 2021). "Redefining differential roles of MAO-A in dopamine degradation and MAO-B in tonic GABA synthesis". Experimental & Molecular Medicine. 53 (7): 1148–1158. doi:10.1038/s12276-021-00646-3. PMC 8333267. PMID 34244591.
- ^ a b c d e f g h i j k l m n o Knoll J (2001). "Antiaging compounds: (-)deprenyl (selegeline) and (-)1-(benzofuran-2-yl)-2-propylaminopentane, [(-)BPAP], a selective highly potent enhancer of the impulse propagation mediated release of catecholamine and serotonin in the brain". CNS Drug Rev. 7 (3): 317–345. doi:10.1111/j.1527-3458.2001.tb00202.x. PMC 6494119. PMID 11607046.
We need to start slowing the age-related functional decline of the striatal dopaminergic neurons in due time. For this reason it is advisable to begin the prophylactic administration of an enhancer substance, for example (–)deprenyl, 1 mg/day, as soon as sexual maturity is reached and the postdevelopmental period of life has just started.
- ^ a b c d e f Shimazu S, Miklya I (May 2004). "Pharmacological studies with endogenous enhancer substances: beta-phenylethylamine, tryptamine, and their synthetic derivatives". Prog Neuropsychopharmacol Biol Psychiatry. 28 (3): 421–427. doi:10.1016/j.pnpbp.2003.11.016. PMID 15093948.
- ^ a b c d e Knoll J (2012). How Selegiline ((-)-Deprenyl) Slows Brain Aging. Bentham Science Publishers. pp. 16, 43, 70, 90. ISBN 978-1-60805-470-1. Retrieved July 4, 2024.
- ^ a b c Harsing LG, Knoll J, Miklya I (August 2022). "Enhancer Regulation of Dopaminergic Neurochemical Transmission in the Striatum". Int J Mol Sci. 23 (15): 8543. doi:10.3390/ijms23158543. PMC 9369307. PMID 35955676.
- ^ a b Pei Y, Asif-Malik A, Canales JJ (2016). "Trace Amines and the Trace Amine-Associated Receptor 1: Pharmacology, Neurochemistry, and Clinical Implications". Front Neurosci. 10: 148. doi:10.3389/fnins.2016.00148. PMC 4820462. PMID 27092049.
- ^ a b Rutigliano G, Accorroni A, Zucchi R (2017). "The Case for TAAR1 as a Modulator of Central Nervous System Function". Front Pharmacol. 8: 987. doi:10.3389/fphar.2017.00987. PMC 5767590. PMID 29375386.
- ^ Sotnikova TD, Caron MG, Gainetdinov RR (August 2009). "Trace amine-associated receptors as emerging therapeutic targets". Mol Pharmacol. 76 (2): 229–235. doi:10.1124/mol.109.055970. PMC 2713119. PMID 19389919.
- ^ Reese EA, Norimatsu Y, Grandy MS, Suchland KL, Bunzow JR, Grandy DK (January 2014). "Exploring the determinants of trace amine-associated receptor 1's functional selectivity for the stereoisomers of amphetamine and methamphetamine". J Med Chem. 57 (2): 378–390. doi:10.1021/jm401316v. PMID 24354319.
- ^ "Levmetamfetamine". PubChem. National Center for Biotechnology Information, U.S. National Library of Medicine. Archived from the original on October 18, 2014. Retrieved October 17, 2014.
- ^ a b Miklya I (June 2014). "Essential difference between the pharmacological spectrum of (-)-deprenyl and rasagiline". Pharmacol Rep. 66 (3): 453–458. doi:10.1016/j.pharep.2013.11.003. PMID 24905523.
- ^ a b Brady LS, Lisanby SH, Gordon JA (2023). "New directions in psychiatric drug development: promising therapeutics in the pipeline". Expert Opin Drug Discov. 18 (8): 835–850. doi:10.1080/17460441.2023.2224555. PMID 37352473.
- ^ a b Kuvarzin SR, Sukhanov I, Onokhin K, Zakharov K, Gainetdinov RR (July 2023). "Unlocking the Therapeutic Potential of Ulotaront as a Trace Amine-Associated Receptor 1 Agonist for Neuropsychiatric Disorders". Biomedicines. 11 (7): 1977. doi:10.3390/biomedicines11071977. PMC 10377193. PMID 37509616.
- ^ a b Dalló J, Lekka N, Knoll J (1986). "The ejaculatory behavior of sexually sluggish male rats treated with (-)deprenyl, apomorphine, bromocriptine and amphetamine". Pol J Pharmacol Pharm. 38 (3): 251–255. PMID 3095802.
- ^ a b Yen TT, Dalló J, Knoll J (1982). "The aphrodisiac effect of low doses of (-) deprenyl in male rats". Pol J Pharmacol Pharm. 34 (5–6): 303–308. PMID 6821215.
- ^ a b Knoll J, Dallo J, Yen TT (1989). "Striatal dopamine, sexual activity and lifespan. Longevity of rats treated with (-)deprenyl". Life Sci. 45 (6): 525–531. doi:10.1016/0024-3205(89)90103-3. PMID 2505007.
- ^ Chambers KC, Phoenix CH (August 1989). "Apomorphine, deprenyl, and yohimbine fail to increase sexual behavior in rhesus males". Behav Neurosci. 103 (4): 816–823. doi:10.1037/0735-7044.103.4.816. PMID 2504225.
- ^ a b Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (January 2001). "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse. 39 (1): 32–41. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707.
- ^ a b c Kohut SJ, Jacobs DS, Rothman RB, Partilla JS, Bergman J, Blough BE (December 2017). "Cocaine-like discriminative stimulus effects of "norepinephrine-preferring" monoamine releasers: time course and interaction studies in rhesus monkeys". Psychopharmacology (Berl). 234 (23–24): 3455–3465. doi:10.1007/s00213-017-4731-5. PMC 5747253. PMID 28889212.
In the present experiments, two monoamine releasers, l-MA and PAL-329, were shown to produce cocaine-like discriminative-stimulus effects in monkeys, suggesting that they meet the above criteria. One of these compounds, l-MA, also has been shown to serve as a positive reinforcer in rodents (Yokel and Pickens 1973) and monkeys (Winger et al 1994), further confirming the overlap with behavioral effects of cocaine. Both compounds also exhibit an approximately 15-fold greater potency in releasing NE than DA, which may be therapeutically advantageous. For example, the subjective effects of l-MA in human studies are similar in some respects to those of d-MA. However, the subjective effects of the two isomers also differ in potentially important ways. While both l-MA and d-MA produce subjective ratings of "drug liking" and "good effects" in experienced stimulant users, only lMA produces concomitant ratings of bad or aversive drug effects (Mendelson et al 2006), a factor which may limit its abuse liability.
- ^ a b c d Mendelson J, Uemura N, Harris D, Nath RP, Fernandez E, Jacob P, Everhart ET, Jones RT (October 2006). "Human pharmacology of the methamphetamine stereoisomers". Clin Pharmacol Ther. 80 (4): 403–420. doi:10.1016/j.clpt.2006.06.013. PMID 17015058.
The stereoisomers of methamphetamine produce markedly different dopamine, norepinephrine, and serotonin responses in various brain regions in rats.41,42 d-Methamphetamine (2 mg/kg) is more potent in releasing caudate dopamine than l-methamphetamine (12 and 18 mg/kg). By use of in vitro uptake and release assays, d-methamphetamine (50% effective concentration [EC50], 24.5 ± 2.1 nmol/L) was 17 times more potent in releasing dopamine than l-methamphetamine (EC50, 416 ± 20 nmol/L) and significantly more potent in blocking dopamine uptake (inhibition constant [Ki ], 114 ± 11 nm versus 4840 ± 178 nm).12,13
- ^ Taylor KM, Snyder SH (June 1970). "Amphetamine: differentiation by d and l isomers of behavior involving brain norepinephrine or dopamine". Science. 168 (3938): 1487–1489. Bibcode:1970Sci...168.1487T. doi:10.1126/science.168.3938.1487. PMID 5463064.
- ^ a b c Fernandez HH, Chen JJ (December 2007). "Monoamine oxidase-B inhibition in the treatment of Parkinson's disease". Pharmacotherapy. 27 (12 Pt 2): 174S–185S. doi:10.1592/phco.27.12part2.174S. PMID 18041937.
These safety issues have been attributed in part to the amphetamine metabolites of selegiline and potential cardiovascular effects.50 [...] Cardiovascular adverse events in these studies may be attributed at least in part to a toxic effect of selegiline's amphetamine metabolites.
- ^ Müller T, Hoffmann JA, Dimpfel W, Oehlwein C (May 2013). "Switch from selegiline to rasagiline is beneficial in patients with Parkinson's disease". J Neural Transm (Vienna). 120 (5): 761–765. doi:10.1007/s00702-012-0927-3. PMID 23196982.
- ^ Rinaldi D, Alborghetti M, Bianchini E, Sforza M, Galli S, Pontieri FE (2023). "Monoamine-oxidase Type B Inhibitors and Cognitive Functions in Parkinson's Disease: Beyond the Primary Mechanism of Action". Curr Neuropharmacol. 21 (5): 1214–1223. doi:10.2174/1570159X20666220905102144. PMC 10286595. PMID 36065929.
[Selegiline] is metabolized into the liver in desmethylselegiline and methamphetamine, which are in turn transformed into amphetamine [45]. [...] there is initial evidence supporting the preferential benefit of selegiline on arousal [46, 70], an effect probably mediated through the stimulant actions of amphetamine-like metabolites of this drug.
- ^ Engberg G, Elebring T, Nissbrandt H (November 1991). "Deprenyl (selegiline), a selective MAO-B inhibitor with active metabolites; effects on locomotor activity, dopaminergic neurotransmission and firing rate of nigral dopamine neurons". The Journal of Pharmacology and Experimental Therapeutics. 259 (2): 841–847. PMID 1658311.
- ^ Bundgaard C, Montezinho LP, Anderson N, Thomsen C, Mørk A (2016). "Selegiline induces a wake promoting effect in rats which is related to formation of its active metabolites". Pharmacol Biochem Behav. 150–151: 147–152. doi:10.1016/j.pbb.2016.10.003. PMID 27984094.
- ^ a b c Barkholtz HM, Hadzima R, Miles A (July 2023). "Pharmacology of R-(-)-Methamphetamine in Humans: A Systematic Review of the Literature". ACS Pharmacol Transl Sci. 6 (7): 914–924. doi:10.1021/acsptsci.3c00019. PMC 10353062. PMID 37470013.
- ^ a b Li L, Lopez JC, Galloway GP, Baggott MJ, Everhart T, Mendelson J (August 2010). "Estimating the intake of abused methamphetamines using experimenter-administered deuterium labeled R-methamphetamine: selection of the R-methamphetamine dose". Ther Drug Monit. 32 (4): 504–507. doi:10.1097/FTD.0b013e3181db82f2. PMC 3040572. PMID 20592647.
- ^ a b Silverstone T, Wells B (1980). "Clinical Psychopharmacology of Amphetamine and Related Compounds". Amphetamines and Related Stimulants: Chemical, Biological, Clinical, and Sociological Aspects. CRC Press. pp. 147–160. doi:10.1201/9780429279843-10. ISBN 978-0-429-27984-3.
A comparison of dextroamphetamine and levoamphetamine revealed that the dextrorotatory isomer was the more potent in elevating mood in normal subjects, being at least twice as potent as the levo form.35 [...] Narcolepsy was one of the first conditions to be treated successfully with amphetamine3 and remains one of the few (some would say the only) clinical indications for its use. While the required oral dose of dextroamphetamine (Dexedrine®) ranges from 5 to 120 mg/day, most patients respond to 10 mg two to four times daily. [...] The closely related compound methylphenidate (Ritalin®), 20 mg two to four times daily, has been shown to be as effective as dextroamphetamine but with less likelihood of causing side effects.61 The same is true of levoamphetamine.62 [...] Nevertheless, as amphetamine has an action on dopaminergic pathways it was considered worthwhile to examine the effects of amphetamine under controlled conditions.95 Twenty patients, all on other anti-Parkinsonian drugs, were studied. There was some subjective improvement in a proportion (less than half) of the patients when they received either dextroamphetamine or levoamphetamine, but there was little objective improvement. The authors remarked that amphetamine was unlikely to have worked anyway in Parkinson's disease as it acts mainly by releasing dopamine and noradrenaline from presynaptic neurons; as the underlying pathology involves a reduction of presynaptic dopamine, there would be insufficient dopamine for amphetamine to release.
- ^ Parkes JD, Fenton GW (December 1973). "Levo(-) amphetamine and dextro(+) amphetamine in the treatment of narcolepsy". J Neurol Neurosurg Psychiatry. 36 (6): 1076–1081. doi:10.1136/jnnp.36.6.1076. PMC 1083612. PMID 4359162.
- ^ a b c Parkes JD, Tarsy D, Marsden CD, Bovill KT, Phipps JA, Rose P, Asselman P (March 1975). "Amphetamines in the treatment of Parkinson's disease". J Neurol Neurosurg Psychiatry. 38 (3): 232–237. doi:10.1136/jnnp.38.3.232. PMC 491901. PMID 1097600.
- ^ Smith RC, Davis JM (June 1977). "Comparative effects of d-amphetamine, l-amphetamine, and methylphenidate on mood in man". Psychopharmacology (Berl). 53 (1): 1–12. doi:10.1007/BF00426687. PMID 407607.
- ^ a b Elsworth JD, Sandler M, Lees AJ, Ward C, Stern GM (1982). "The contribution of amphetamine metabolites of (-)-deprenyl to its antiparkinsonian properties". J Neural Transm. 54 (1–2): 105–110. doi:10.1007/BF01249283. PMID 6809891.
- ^ a b Dezsi L, Vecsei L (2017). "Monoamine Oxidase B Inhibitors in Parkinson's Disease". CNS Neurol Disord Drug Targets. 16 (4): 425–439. doi:10.2174/1871527316666170124165222. PMID 28124620.
- ^ a b c d e f Knoll J (1995). "Rationale for (-)deprenyl (selegiline) medication in Parkinson's disease and in prevention of age-related nigral changes". Biomed Pharmacother. 49 (4): 187–195. doi:10.1016/0753-3322(96)82619-9. PMID 7669938.
We propose that the healthy population be maintained on 10-15 mg (-)deprenyl weekly starting at age 45 in order to combat the age-related decline of the nigrostriatal dopaminergic neurons. Prophylactic (-)deprenyl medication seems to offer a reasonable prospect of improving the quality of life in the later decades, delaying the time of natural death and decreasing the susceptibility of age-related neurological diseases. like Parkinson's diesase and Alzheimer's disease.
- ^ a b c d e f Knoll J (1993). "The Pharmacological Basis of the Therapeutic Effect of (—)-Deprenyl in Age-Related Neurological Diseases". Inhibitors of Monoamine Oxidase B: Pharmacology and Clinical Use in Neurodegenerative Disorders. Milestones in Drug Therapy. Basel: Birkhäuser Basel. pp. 145–168. doi:10.1007/978-3-0348-6348-3_7. ISBN 978-3-0348-6349-0.
It is concluded that in Parkinson's disease and Alzheimer's disease patients need to be treated daily with 10 mg (-)deprenyl from diagnosis until death, irrespective of other medication. The healthy population is proposed to be maintained on 10-15 mg (-)deprenyl weekly from age 45 in order to fight against the age-related decline of the nigrostriatal dopaminergic neurons, the hitherto known most rapidly aging neurons in the brain. Prophylactic deprenyl medication seems to bid fair prospects, on the one hand, to an improved quality of life in the latter decades with hopes to a shift in the time of natural death, and on the other hand, to a decreased susceptibility to agerelated neurological diseases.
- ^ a b c d e Gerlach M, Riederer P (1993). "The Pathophysiological Basis of Parkinson's Disease". Inhibitors of Monoamine Oxidase B: Pharmacology and Clinical Use in Neurodegenerative Disorders (in German). Basel: Birkhäuser Basel. p. 25–50. doi:10.1007/978-3-0348-6348-3_2. ISBN 978-3-0348-6349-0.
- ^ Riederer P, Wuketich S (1976). "Time course of nigrostriatal degeneration in parkinson's disease. A detailed study of influential factors in human brain amine analysis". J Neural Transm. 38 (3–4): 277–301. doi:10.1007/BF01249445. PMID 956814.
- ^ Itzhak Y (1994). Sigma Receptors. Academic Press. pp. 84–. ISBN 978-0-12-376350-1.
- ^ Stone TW (1993). Acetylcholine, Sigma Receptors, CCK and Eicosanoids, Neurotoxins. Taylor & Francis. pp. 124–. ISBN 978-0-7484-0063-8.
- ^ Azzaro AJ, Ziemniak J, Kemper E, Campbell BJ, VanDenBerg C (October 2007). "Pharmacokinetics and absolute bioavailability of selegiline following treatment of healthy subjects with the selegiline transdermal system (6 mg/24 h): a comparison with oral selegiline capsules". J Clin Pharmacol. 47 (10): 1256–1267. doi:10.1177/0091270007304779. PMID 17715422.
- ^ Paz-Ramos MI, Cruz SL, Violante-Soria V (2023). "Amphetamine-type Stimulants: Novel Insights into their Actions and use Patterns". Rev Invest Clin. 75 (3): 143–157. doi:10.24875/RIC.23000110. PMID 37441770.
- ^ Barrett JS, Szego P, Rohatagi S, Morales RJ, De Witt KE, Rajewski G, Ireland J (October 1996). "Absorption and presystemic metabolism of selegiline hydrochloride at different regions in the gastrointestinal tract in healthy males". Pharmaceutical Research. 13 (10): 1535–1540. doi:10.1023/A:1016035730754. PMID 8899847. S2CID 24654277.
- ^ a b Kalász H, Magyar K, Szőke É, Adeghate E, Adem A, Hasan MY, Nurulain SM, Tekes K (2014). "Metabolism of selegiline [(-)-deprenyl)]". Curr Med Chem. 21 (13): 1522–1530. doi:10.2174/0929867321666131218094352. PMID 24350849.
- ^ Musshoff F (February 2000). "Illegal or legitimate use? Precursor compounds to amphetamine and methamphetamine". Drug Metab Rev. 32 (1): 15–44. doi:10.1081/dmr-100100562. PMID 10711406.
- ^ Cody JT (May 2002). "Precursor medications as a source of methamphetamine and/or amphetamine positive drug testing results". J Occup Environ Med. 44 (5): 435–450. doi:10.1097/00043764-200205000-00012. PMID 12024689.
- ^ DE 1568277, Ecsery Z, Kosa I, Knoll J, Somfai E, "Verfahren zur Herstellung von neuen,optisch aktiven Phenylisopylamin-Derivaten [Process for the preparation of new, optically active phenylisopylamine derivatives]", published 1970-04-30, assigned to Chinoin Gyógyszer-és Vegyészeti Termékek Gyára RT
- ^ J. Hermann Nee Voeroes, Z. Ecsery, G. Sabo, L. Arvai, L. Nagi, O. Orban, E. Sanfai, U.S. patent 4,564,706 (1986)
- ^ EP 344675, Hájicek J, Hrbata J, Pihera P, Brunová B, Ferenc M, Krepelka J, Kvapil L, Pospisil J, "Method for the production of selegiline hydrochloride", published 989-12-06, assigned to SPOFA Spojené Podniky Pro Zdravotnickou Vyrobu
- ^ Fowler JS (July 1977). "2-Methyl-3-butyn-2-ol as an acetylene precursor in the Mannich reaction. A new synthesis of suicide inactivators of monoamine oxidase". The Journal of Organic Chemistry. 42 (15): 2637–2639. doi:10.1021/jo00435a026. PMID 874623.
- ^ Zeller EA, Barsky J (November 1952). "In vivo inhibition of liver and brain monoamine oxidase by 1-Isonicotinyl-2-isopropyl hydrazine". Proc Soc Exp Biol Med. 81 (2): 459–461. doi:10.3181/00379727-81-19910. PMID 13027339.
- ^ "Sanofi Extends Holding in Chinoin". The Pharma Letter. September 19, 1993.
- ^ a b c d Knoll J, Ecseri Z, Kelemen K, Nievel J, Knoll B (May 1965). "Phenylisopropylmethylpropinylamine (E-250), a new spectrum psychic energizer". Archives Internationales de Pharmacodynamie et de Therapie. 155 (1): 154–164. PMID 4378644.
- ^ Bryant JM, Torosdag S, Schvartz N, Fletcher L, Fertig H, Schwartz MS, Quan RB (October 1961). "Antihypertensive properties of pargyline hydrochloride. New non-hydrazine monoamine oxidase inhibitor compared with sulphonamide diuretics". JAMA. 178: 406–409. doi:10.1001/jama.1961.73040430005010. PMID 13874134.
- ^ Magyar K, Vizi ES, Ecseri Z, Knoll J (1967). "Comparative pharmacological analysis of the optical isomers of phenyl-isopropyl-methyl-propinylamine (E-250)". Acta Physiologica Academiae Scientiarum Hungaricae. 32 (4): 377–387. PMID 5595908.
- ^ a b Healy D (2000). "The psychopharmacology of life and death. Interview with Joseph Knoll.". The Psychopharmacologists, Vol. III: Interviews. London: Arnold. pp. 81–110. ISBN 978-0-340-76110-6.
- ^ Johnston JP (July 1968). "Some observations upon a new inhibitor of monoamine oxidase in brain tissue". Biochem Pharmacol. 17 (7): 1285–1297. doi:10.1016/0006-2952(68)90066-x. PMID 5659776.
- ^ Knoll J, Magyar K (1972). "Some puzzling pharmacological effects of monoamine oxidase inhibitors". Adv Biochem Psychopharmacol. 5: 393–408. PMID 5066229.
- ^ Knoll J, Vizi ES, Somogyi G (1968). "Phenylisopropylmethylpropynylamine (E-250), a Monoaminooxidase Inhibitor Antagonising Effects of Tyramine". Arzneimittel-Forschung. 18 (1): 109–112.
The compound E250 has a property not known in other MAOIs, namely its inhibiting of the effects of tyramine. E250 inhibits the pressor responses of cats and rabbits to tyramine, but has no effect on isolated vas deferens preparations. In view of the known cheese reaction (clinical symptoms associated with consumption of cheese caused by MAOI potentiation of tyramine), this property of E250 may be of importance in therapy.
- ^ Varga E (1965). "Vorläufiger Bericht über die Wirkung des Präparates E-250 (phenyl-isopropyl-methyl-propinylamine-chlorhydrat)". III Conferentia Hungarica pro Therapia et Investigatione in Pharmacologia. Budapest: Publishing House of the Hungarian Academy of Sciences. pp. 197–201.
- ^ Varga E, Tringer L (1967). "Clinical trial of a new type promptly acting psychoenergetic agent (phenyl-isopropyl-methylpropinyl-HCl, "E-250")". Acta Med Acad Sci Hung. 23 (3): 289–295. PMID 6056555.
- ^ Tringer L, Haits G, Varga E (1971). "The effect of (-) E-250,(-) L-phenyl-isopropylmethylpropinyl-amine HCl, in depression". V. Conferentia Hungarica pro Therapia et Investigatione in Pharmacologia. pp. 111–114.
- ^ Mann J, Gershon S (March 1980). "L-deprenyl, a selective monoamine oxidase type-B inhibitor in endogenous depression". Life Sci. 26 (11): 877–882. doi:10.1016/0024-3205(80)90350-1. PMID 6768943.
- ^ a b Birkmayer W, Riederer P, Linauer W, Knoll J (1984). "L-deprenyl plus L-phenylalanine in the treatment of depression". J Neural Transm. 59 (1): 81–87. doi:10.1007/BF01249880. PMID 6425455.
- ^ Birkmayer W, Riederer P, Youdim MB, Linauer W (1975). "The potentiation of the anti akinetic effect after L-dopa treatment by an inhibitor of MAO-B, Deprenil". Journal of Neural Transmission. 36 (3–4): 303–326. doi:10.1007/BF01253131. PMID 1172524. S2CID 38179089. Archived from the original on February 12, 2013.
- ^ a b Cromie WJ (November 7, 2002). "Bodkin is Patching up Depression". Harvard University Gazette. Retrieved September 8, 2007.
- ^ Reynolds GP, Elsworth JD, Blau K, Sandler M, Lees AJ, Stern GM (December 1978). "Deprenyl is metabolized to methamphetamine and amphetamine in man". Br J Clin Pharmacol. 6 (6): 542–544. doi:10.1111/j.1365-2125.1978.tb00883.x. PMC 1429688. PMID 728327.
- ^ Knoll J, Miklya I (1994). "Multiple, small dose administration of (-)deprenyl enhances catecholaminergic activity and diminishes serotoninergic activity in the brain and these effects are unrelated to MAO-B inhibition". Arch Int Pharmacodyn Ther. 328 (1): 1–15. PMID 7893186.
- ^ Knoll J, Miklya I, Knoll B, Markó R, Kelemen K (1996). "(-)Deprenyl and (-)1-phenyl-2-propylaminopentane, [(-)PPAP], act primarily as potent stimulants of action potential-transmitter release coupling in the catecholaminergic neurons". Life Sci. 58 (10): 817–827. doi:10.1016/0024-3205(96)00014-8. PMID 8602114.
- ^ Knoll J, Knoll B, Török Z, Timár J, Yasar S (1992). "The pharmacology of 1-phenyl-2-propylamino-pentane (PPAP), a deprenyl-derived new spectrum psychostimulant". Arch Int Pharmacodyn Ther. 316: 5–29. PMID 1356324.
- ^ Knoll J, Yoneda F, Knoll B, Ohde H, Miklya I (December 1999). "(-)1-(Benzofuran-2-yl)-2-propylaminopentane, [(-)BPAP], a selective enhancer of the impulse propagation mediated release of catecholamines and serotonin in the brain". Br J Pharmacol. 128 (8): 1723–1732. doi:10.1038/sj.bjp.0702995. PMC 1571822. PMID 10588928.
- ^ Gaszner P, Miklya I (December 2004). "The use of the synthetic enhancer substances (-)-deprenyl and (-)-BPAP in major depression". Neuropsychopharmacol Hung. 6 (4): 210–220. PMID 15825677.
- ^ Knoll J (August 2003). "Enhancer regulation/endogenous and synthetic enhancer compounds: a neurochemical concept of the innate and acquired drives". Neurochem Res. 28 (8): 1275–1297. doi:10.1023/a:1024224311289. PMID 12834268.
- ^ Frampton JE, Plosker GL (2007). "Selegiline transdermal system: in the treatment of major depressive disorder". Drugs. 67 (2): 257–265, discussion 266–267. doi:10.2165/00003495-200767020-00006. PMID 17284087. S2CID 42425086.
- ^ Duffy M (December 3, 2002). "Patch Raises New Hope For Beating Depression". The New York Times. ISSN 0362-4331.
- ^ Cascade EF, Kalali AH, Preskorn SH (June 2007). "Emsam: the first year". Psychiatry. 4 (6): 19–21. PMC 2921248. PMID 20711332.
- ^ a b c Elks J (1990). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. p. 441. ISBN 978-1-4757-2085-3. Retrieved July 4, 2024.
- ^ a b Morton IK, Hall JM (1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Netherlands. p. 254. ISBN 978-94-011-4439-1. Retrieved July 4, 2024.
selegiline (BAN, INN) (selegiline hydrochloride [USAN]; l-deprenyl; Deprenaline™; Eldepryl™) is an acetylenic derivative of phenethylamine, and the (R)-form (laevorotatory) is the pharmacologically active isomer. It has (type B) monoaminde-oxidase inhibitor activity. and can be used as an antiparkinsonian, as an enzyme inhibitor it prolongs the action of endogenous dopamine, or it can be administered in combination with levodopa. It was once believed to be an ANTIDEPRESSANT but is now thought that specific type-B MAO inhibitors are not antidepressants. They produce much less adverse interactions with foodstuffs.
- ^ a b c d Schweizerischer Apotheker-Verein (2000). Index Nominum 2000: International Drug Directory. Medpharm Scientific Publishers. p. 939. ISBN 978-3-88763-075-1. Retrieved July 4, 2024.
- ^ Feinberg S (November 1, 2006). "EMSAM: A User-Friendly MAOI?". CARLAT PUBLISHING. Retrieved July 4, 2024.
Let us begin with a question that has been on everybody's mind: Why is it named "EMSAM?" It required several phone calls to Bristol-Myers Squibb, but we finally have our answer. Somerset Pharmaceuticals, the original developer of the formulation, held a company-wide contest to see who could come up with the best name for their new product. One employee offered a name that combined the names of his two children, Emily and Sam. The company vetted the name through its branding consultants and focus groups, eventually deciding that EMSAM (in all caps, for unclear reasons) was the winner. We hope the children will get a cut of the profits!
- ^ Dorsey ER, Thompson JP, Dayoub EJ, George B, Saubermann LA, Holloway RG (July 2009). "Selegiline shortage: Causes and costs of a generic drug shortage". Neurology. 73 (3): 213–217. doi:10.1212/WNL.0b013e3181ae7b04. PMC 2715573. PMID 19620609.
- ^ Pearce D (1995). The Hedonistic Imperative. OCLC 44325836.
- ^ "Sam Barker and David Pearce on Art, Paradise Engineering, and Existential Hope (With Guest Mix) | The FLI Podcast". Future of Life Institute (audio, transcript). June 24, 2020.
- ^ a b Murphy HT (December 14, 2022). "Sam Bankman-Fried Confirmed He Wears an Emsam Patch. What's an Emsam Patch?". Slate Magazine. Retrieved July 2, 2024.
- ^ a b Sigalos M (August 14, 2023). "Sam Bankman-Fried wins approval to receive Adderall for ADHD while in jail". CNBC. Retrieved July 2, 2024.
- ^ Alexander S (November 16, 2022). "The Psychopharmacology Of The FTX Crash". Astral Codex Ten. Retrieved July 4, 2024.
Going back to @AutismCapital's picture: [...] What's the blue-green bottle to the left of the red circle? Here the detectives on r/NootropicsDepot recognized it as their company's old brand of adrafinil7. Adrafinil is a prodrug of modafinil, an unusual stimulant-like drug. That is, your body metabolizes adrafinil and turns it into modafinil after you take it. So was SBF effectively on modafinil? Seems likely - many traders are. I won't lie - modafinil is a good stimulant, during medical residency some doctors (including me) would use it to stay alert through the night shift. It's not any better than Adderall or anything, just a bit different and easier to get.
- ^ Hurwitz G (2019). Out of the dark. Penguin Books. p. 431. ISBN 9780718185480.
- ^ Lidsky T, Schneider J (2010). Brain Candy: Boost Your Brain Power with Vitamins, Supplements, Drugs, and Other Substance. Touchstone. pp. 89–93. ISBN 978-0-7432-1843-6. Retrieved July 5, 2024.
DEPRENYL, ELDEPRYL, OR SELEGILINE: Prescription drug; available in pharmacies by prescription and without prescription through Internet suppliers. [...]
- ^ Saunders N, Heron L (1993). E for Ecstasy. London: N. Saunders. ISBN 978-0-9501628-8-1. OCLC 29388575.[page needed]
- ^ Saunders N. "Test results of 30 samples of Ecstasy bought in British clubs between 11/94 and 7/95".
- ^ a b c Docherty JR (June 2008). "Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA)". Br J Pharmacol. 154 (3): 606–622. doi:10.1038/bjp.2008.124. PMC 2439527. PMID 18500382.
- ^ Ferdinandy P, Yoneda F, Muraoka S, Fürst S, Gyires K, Miklya I (February 2020). "Geroprotection in the future. In memoriam of Joseph Knoll: The selegiline story continues". European Journal of Pharmacology. 868: 172793. doi:10.1016/j.ejphar.2019.172793. PMID 31743738. S2CID 208185366.
- ^ Knoll J, Miklya I (December 2016). "Longevity study with low doses of selegiline/(-)-deprenyl and (2R)-1-(1-benzofuran-2-yl)-N-propylpentane-2-amine (BPAP)". Life Sciences. 167: 32–38. doi:10.1016/j.lfs.2016.10.023. PMID 27777099.
- ^ Susanna Furst (2018). "In memoriam Joseph Knoll (1925-2018) | Hungarian Society for Experimental and Clinical Pharmacology". Retrieved April 10, 2023.
- ^ Froestl W, Muhs A, Pfeifer A (2014). "Cognitive enhancers (nootropics). Part 2: drugs interacting with enzymes. Update 2014". J Alzheimers Dis. 42 (1): 1–68. doi:10.3233/JAD-140402. PMID 24903780.
Selegiline reversed Aβ25-35-induced cognitive 2252 deficits in male mice [617].
- ^ Carageorgiou H, Sideris AC, Messari I, Liakou CI, Tsakiris S (August 2008). "The effects of rivastigmine plus selegiline on brain acetylcholinesterase, (Na, K)-, Mg-ATPase activities, antioxidant status, and learning performance of aged rats". Neuropsychiatric Disease and Treatment. 4 (4): 687–699. doi:10.2147/ndt.s3272. PMC 2536534. PMID 19043511.
- ^ Stoll S, Hafner U, Pohl O, Müller WE (1994). "Age-related memory decline and longevity under treatment with selegiline". Life Sciences. 55 (25–26): 2155–2163. doi:10.1016/0024-3205(94)00396-3. PMID 7997074.
- ^ Puurunen K, Jolkkonen J, Sirviö J, Haapalinna A, Sivenius J (February 2001). "Selegiline combined with enriched-environment housing attenuates spatial learning deficits following focal cerebral ischemia in rats". Experimental Neurology. 167 (2): 348–355. doi:10.1006/exnr.2000.7563. PMID 11161623. S2CID 22769187.
- ^ Sabelli HC (March 1991). "Rapid treatment of depression with selegiline-phenylalanine combination". J Clin Psychiatry. 52 (3): 137. PMID 1900832.
- ^ Sabelli H, Fink P, Fawcett J, Tom C (1996). "Sustained antidepressant effect of PEA replacement". J Neuropsychiatry Clin Neurosci. 8 (2): 168–71. doi:10.1176/jnp.8.2.168. PMID 9081552.
- ^ a b Simpson HB, Schneier FR, Marshall RD, Campeas RB, Vermes D, Silvestre J, Davies S, Liebowitz MR (1998). "Low dose selegiline (L-Deprenyl) in social phobia". Depress Anxiety. 7 (3): 126–129. doi:10.1002/(SICI)1520-6394(1998)7:3<126::AID-DA5>3.0.CO;2-9. PMID 9656093.
- ^ Padilha SC, Virtuoso S, Tonin FS, Borba HH, Pontarolo R (October 2018). "Efficacy and safety of drugs for attention deficit hyperactivity disorder in children and adolescents: a network meta-analysis". European Child & Adolescent Psychiatry. 27 (10): 1335–1345. doi:10.1007/s00787-018-1125-0. PMID 29460165. S2CID 3402756.
- ^ a b Buoli M, Serati M, Cahn W (2016). "Alternative pharmacological strategies for adult ADHD treatment: a systematic review". Expert Review of Neurotherapeutics. 16 (2): 131–144. doi:10.1586/14737175.2016.1135735. PMID 26693882. S2CID 33004517.
- ^ Bloch MH, Panza KE, Landeros-Weisenberger A, Leckman JF (September 2009). "Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders". J Am Acad Child Adolesc Psychiatry. 48 (9): 884–893. doi:10.1097/CHI.0b013e3181b26e9f. PMC 3943246. PMID 19625978.
- ^ Rubinstein S, Malone MA, Roberts W, Logan WJ (August 2006). "Placebo-controlled study examining effects of selegiline in children with attention-deficit/hyperactivity disorder". Journal of Child and Adolescent Psychopharmacology. 16 (4): 404–415. doi:10.1089/cap.2006.16.404. PMID 16958566.
- ^ Akhondzadeh S, Tavakolian R, Davari-Ashtiani R, Arabgol F, Amini H (August 2003). "Selegiline in the treatment of attention deficit hyperactivity disorder in children: a double blind and randomized trial". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 27 (5): 841–845. doi:10.1016/S0278-5846(03)00117-9. PMID 12921918. S2CID 23234928.
- ^ Wilens TE, Spencer TJ, Biederman J (March 2002). "A review of the pharmacotherapy of adults with attention-deficit/hyperactivity disorder". Journal of Attention Disorders. 5 (4): 189–202. doi:10.1177/108705470100500401. PMID 11967475. S2CID 37417459.
- ^ Tcheremissine OV, Salazar JO (June 2008). "Pharmacotherapy of adult attention deficit/hyperactivity disorder: review of evidence-based practices and future directions". Expert Opinion on Pharmacotherapy. 9 (8): 1299–1310. doi:10.1517/14656566.9.8.1299. PMID 18473705. S2CID 73193888.
- ^ a b Mechcatie E (July 2003). "Transdermal MAO inhibitor patch effective for ADHD". Clinical Psychiatry News.
- ^ a b Hailwood JM (September 27, 2018). Novel approaches towards pharmacological enhancement of motivation (Thesis). University of Cambridge. pp. 13–14. doi:10.17863/CAM.40216.
Several studies have investigated the effects of monoamine-oxidase (MAO) inhibitors on effort expenditure in rodents. The MAO-B inhibitor deprenyl (also known as selegiline) is used as a treatment in Parkinson's disease and depression (Miklya 2016) Although it is not clear whether administration of deprenyl is sufficient for an increase extracellular DA levels (Kato et al. 1986; Butcher et al. 1990; Yohn et al. 2018), the compound does potentiate stimulant induced DA release (Schiffer et al. 2003). Acute administration of deprenyl has been shown to partially rescue a tetrabenazine-induced deficit on both ERC and PR choice tasks (Randall, Lee, Nunes, et al. 2014; Contreras-Mora et al. 2018). Deprenyl is also able to facilitate high effort selection on a PR choice task in intact rats (Yohn et al. 2018). Together, these studies suggest that DAT inhibition as well as MAO-B inhibitors can increase effort expenditure in intact rodents as well as reverse pharmacological-induced motivational deficits.
- ^ a b Callaghan CK, Rouine J, O'Mara SM (2018). "Potential roles for opioid receptors in motivation and major depressive disorder". The Opioid System as the Interface between the Brain's Cognitive and Motivational Systems. Progress in Brain Research. Vol. 239. pp. 89–119. doi:10.1016/bs.pbr.2018.07.009. ISBN 978-0-444-64167-0. PMID 30314570.
Administration of the monoamine oxidase B (MAO-B) inhibitor, deprenyl, has been shown to reverse the low effort bias or amotivational symptoms induced by TBZ in effort based decision-making tasks (Contreras-Mora et al., 2018). Treatment with the most common antidepressant drugs, SSRIs, fluoxetine or citalopram, does not reverse the effort based effects of TBZ and in fact produced further impairments in lever pressing (Yohn et al., 2016). Administration of a different class of antidepressant therapy, norepinephrine uptake inhibitor, desipramine, did not reverse TBZ effects either (Yohn et al., 2016). Interestingly MAO inhibitors can also be used in the treatment of depression but only irreversible MAO-B inhibitors like deprenyl, and not MAO-A inhibitors, have antidepressant effects in humans and recover TBZ effects in rodents (Contreras-Mora et al., 2018; Jang et al., 2013; Sclar et al., 2013). Further, Parkinson's patients receiving the MAO-B inhibitor, rasagiline, in combination with SSRIs, demonstrated a significant improvement in motivation compared to patients receiving SSRIs alone (Smith et al., 2015). [...] There are currently few therapeutic options for patients suffering from motivational dysfunction. The MAO-B inhibitor, deprenyl, has shown the most potential to reverse the low-effort bias or amotivational symptoms in animal models but has not been tested in humans.
{{cite book}}:|journal=ignored (help) - ^ Yohn SE, Reynolds S, Tripodi G, Correa M, Salamone JD (April 2018). "The monoamine-oxidase B inhibitor deprenyl increases selection of high-effort activity in rats tested on a progressive ratio/chow feeding choice procedure: Implications for treating motivational dysfunctions". Behav Brain Res. 342: 27–34. doi:10.1016/j.bbr.2017.12.039. PMID 29292157.
- ^ Contreras-Mora H, Rowland MA, Yohn SE, Correa M, Salamone JD (March 2018). "Partial reversal of the effort-related motivational effects of tetrabenazine with the MAO-B inhibitor deprenyl (selegiline): Implications for treating motivational dysfunctions". Pharmacol Biochem Behav. 166: 13–20. doi:10.1016/j.pbb.2018.01.001. PMID 29309800.
- ^ van Dalen JW, Moll van Charante EP, Nederkoorn PJ, van Gool WA, Richard E (March 2013). "Poststroke apathy". Stroke. 44 (3): 851–860. doi:10.1161/STROKEAHA.112.674614. PMID 23362076.
- ^ a b Al-Adawi SH (1998). The neuropsychopharmacology of motivation: an examination of reward and frontal-subcortical mechanisms and functions (Thesis). Retrieved July 5, 2024.
Clinically, there are some indications (primarily anecdotal single case studies) that medications that release catecholamines (e.g. amantadine, amphetamine, bupropion, methylphenidate, and selegiline) may relieve symptoms reflecting poverty of action and thought; these include akinetic mutism and extrapyramidal disorders characterised by apathy, diminished spontaneity of speech and movement, difficulty in initiation, neglect, attention deficit and non-fluent aphasia (Muller & Von Cramon, 1994).
- ^ Marin RS, Wilkosz PA (2005). "Disorders of diminished motivation". J Head Trauma Rehabil. 20 (4): 377–88. doi:10.1097/00001199-200507000-00009. PMID 16030444.
- ^ a b Deng X, Shang X, Guo K, Zhou L, Wang Y, Wu Y, Liang S, E F, Liu W, Wang Z, Li X, Yang K (August 2023). "Efficacy and safety of antidepressants for smoking cessation: A systematic review and network meta-analysis". Addict Biol. 28 (8): e13303. doi:10.1111/adb.13303. PMID 37500482.
- ^ a b Hajizadeh A, Howes S, Theodoulou A, Klemperer E, Hartmann-Boyce J, Livingstone-Banks J, Lindson N (May 2023). "Antidepressants for smoking cessation". Cochrane Database Syst Rev. 2023 (5): CD000031. doi:10.1002/14651858.CD000031.pub6. PMC 10207863. PMID 37230961.
- ^ Castells X, Casas M, Pérez-Mañá C, Roncero C, Vidal X, Capellà D (February 2010). Castells X (ed.). "Efficacy of psychostimulant drugs for cocaine dependence". Cochrane Database Syst Rev (2): CD007380. doi:10.1002/14651858.CD007380.pub3. PMID 20166094.
- ^ Costa AM, Lima MS, Mari Jde J (September 2006). "A systematic review on clinical management of antipsychotic-induced sexual dysfunction in schizophrenia". Sao Paulo Med J. 124 (5): 291–297. doi:10.1590/s1516-31802006000500012. PMID 17262163.
- ^ Schmidt HM, Hagen M, Kriston L, Soares-Weiser K, Maayan N, Berner MM (November 2012). "Management of sexual dysfunction due to antipsychotic drug therapy". Cochrane Database Syst Rev. 11 (11): CD003546. doi:10.1002/14651858.CD003546.pub3. PMC 7003677. PMID 23152218.
- ^ a b Murphy BP, Chung YC, Park TW, McGorry PD (December 2006). "Pharmacological treatment of primary negative symptoms in schizophrenia: a systematic review". Schizophr Res. 88 (1–3): 5–25. doi:10.1016/j.schres.2006.07.002. PMID 16930948.
- ^ a b c Maski K, Trotti LM, Kotagal S, Robert Auger R, Swick TJ, Rowley JA, Hashmi SD, Watson NF (September 2021). "Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment". J Clin Sleep Med. 17 (9): 1895–1945. doi:10.5664/jcsm.9326. PMC 8636345. PMID 34743790.
- ^ Annane D, Moore DH, Barnes PR, Miller RG (July 2006). "Psychostimulants for hypersomnia (excessive daytime sleepiness) in myotonic dystrophy". Cochrane Database Syst Rev. 2006 (3): CD003218. doi:10.1002/14651858.CD003218.pub2. PMC 9006877. PMID 16855999.
- ^ a b c d Yeh PG, Spruyt K, DelRosso LM, Walters AS (2023). "A Narrative Review of the Lesser Known Medications for Treatment of Restless Legs Syndrome and Pathogenetic Implications for Their Use". Tremor Other Hyperkinet Mov (N Y). 13: 7. doi:10.5334/tohm.739. PMC 9983500. PMID 36873914.
- ^ a b Aurora RN, Kristo DA, Bista SR, Rowley JA, Zak RS, Casey KR, Lamm CI, Tracy SL, Rosenberg RS (August 2012). "The treatment of restless legs syndrome and periodic limb movement disorder in adults--an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline". Sleep. 35 (8): 1039–1062. doi:10.5665/sleep.1988. PMC 3397811. PMID 22851801.
- ^ a b Grewal M, Hawa R, Shapiro C (March 2002). "Treatment of periodic limb movements in sleep with selegiline HCl". Mov Disord. 17 (2): 398–401. doi:10.1002/mds.10082. PMID 11921131.
- ^ Soares-Weiser K, Rathbone J, Ogawa Y, Shinohara K, Bergman H (March 2018). "Miscellaneous treatments for antipsychotic-induced tardive dyskinesia". Cochrane Database Syst Rev. 2018 (3): CD000208. doi:10.1002/14651858.CD000208.pub2. PMC 6494382. PMID 29552749.
- ^ Wilcock GK, Birks J, Whitehead A, Evans SJ (February 2002). "The effect of selegiline in the treatment of people with Alzheimer's disease: a meta-analysis of published trials". Int J Geriatr Psychiatry. 17 (2): 175–183. doi:10.1002/gps.545. PMID 11813282.
- ^ Sanders O, Rajagopal L (June 2020). "Phosphodiesterase Inhibitors for Alzheimer's Disease: A Systematic Review of Clinical Trials and Epidemiology with a Mechanistic Rationale". J Alzheimers Dis Rep. 4 (1): 185–215. doi:10.3233/ADR-200191. PMC 7369141. PMID 32715279.
- ^ Laver K, Dyer S, Whitehead C, Clemson L, Crotty M (April 2016). "Interventions to delay functional decline in people with dementia: a systematic review of systematic reviews". BMJ Open. 6 (4): e010767. doi:10.1136/bmjopen-2015-010767. PMC 4854009. PMID 27121704.
- ^ Stinton C, McKeith I, Taylor JP, Lafortune L, Mioshi E, Mak E, Cambridge V, Mason J, Thomas A, O'Brien JT (August 2015). "Pharmacological Management of Lewy Body Dementia: A Systematic Review and Meta-Analysis". Am J Psychiatry. 172 (8): 731–742. doi:10.1176/appi.ajp.2015.14121582. PMID 26085043.
- ^ Beghi E, Binder H, Birle C, Bornstein N, Diserens K, Groppa S, Homberg V, Lisnic V, Pugliatti M, Randall G, Saltuari L, Strilciuc S, Vester J, Muresanu D (September 2021). "European Academy of Neurology and European Federation of Neurorehabilitation Societies guideline on pharmacological support in early motor rehabilitation after acute ischaemic stroke". Eur J Neurol. 28 (9): 2831–2845. doi:10.1111/ene.14936. PMID 34152062.
- ^ a b Szymkowicz E, Alnagger N, Seyfzadehdarabad F, Cardone P, Whyte J, Gosseries O (2023). "Pharmacological Treatments". Coma and Disorders of Consciousness (3 ed.). Cham: Springer International Publishing. pp. 115–146. doi:10.1007/978-3-031-50563-8_7. ISBN 978-3-031-50562-1.
Approved by the FDA for the treatment of depression and Parkinson's disease, selegiline is also succinctly investigated off-label for the treatment of DoC [85, 86]. Masotta et al. (2018) administered selegiline (5–10 mg daily for 10 weeks) to 10 neurorehabilitation patients (six UWS and four MCS, mixed etiologies) with contraindications or signifcant adverse effects to amantadine [86]. The clinical diagnosis improved in four out of seven patients, and the arousal level improved in three out of seven patients after treatment and at 1-month follow-up as assessed by the CRS-R (only seven patients who completed the protocol without relevant side effects). The potential adverse effects of selegiline include persistent diarrhea and tachycardia [86].
- ^ a b Masotta O, Trojano L, Loreto V, Moretta P, Estraneo A (November 2018). "Selegiline in Patients With Disorder of Consciousness: An Open Pilot Study". Can J Neurol Sci. 45 (6): 688–691. doi:10.1017/cjn.2018.315. PMID 30430963.
- ^ Knoll J (1989). "The pharmacology of selegiline ((-)deprenyl). New aspects". Acta Neurologica Scandinavica. Supplementum. 126: 83–91. doi:10.1111/j.1600-0404.1989.tb01787.x. PMID 2515725.
(-)Deprenyl is a safe substance, which, when administered for long periods in very small doses, facilitates the activity of the nigrostriatal dopaminergic neurons with high selectivity (7, 8) and protects these neurons from the neurotoxicity of 6-hydroxydopamine (6-OHDA) (9) and 1-methyl-4-phenyl-1, 2, 5, 6-tetrahydropyridine (MPTP) (10).
- ^ Ebadi M, Sharma S, Shavali S, El Refaey H (February 2002). "Neuroprotective actions of selegiline". Journal of Neuroscience Research. 67 (3): 285–289. doi:10.1002/jnr.10148. PMID 11813232.
- ^ a b Bentué-Ferrer D, Ménard G, Allain H (1996). "Monoamine Oxidase B Inhibitors: Current Status and Future Potential". CNS Drugs. 6 (3): 217–236. doi:10.2165/00023210-199606030-00005. ISSN 1172-7047.
Selegiline also protects against the lesions induced by 6-hydroxydopamine (6-0H-DA),[90] This is another aminotoxin, with a mechanism of action involving the formation of hydrogen peroxide and, subsequently, oxygenated radicals. In contrast, selegiline potentiates the neurotoxicity of p-chloroamphetamine on serotonergic neurons.[91]
- ^ Knoll J (1978). "The possible mechanisms of action of (-)deprenyl in Parkinson's disease". Journal of Neural Transmission. 43 (3–4): 177–198. doi:10.1007/BF01246955. PMID 745011.
- ^ Cohen G, Pasik P, Cohen B, Leist A, Mytilineou C, Yahr MD (October 1984). "Pargyline and deprenyl prevent the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in monkeys". European Journal of Pharmacology. 106 (1): 209–210. doi:10.1016/0014-2999(84)90700-3. PMID 6442232.
- ^ Finnegan KT, DeLanney LE, Irwin I, Ricaurte GA, Langston JW (September 1989). "The amine-depleting effects of 5,7-dihydroxytryptamine (5,7-DHT) in C57BL/6 mice do not increase with age". Brain Research. 496 (1–2): 251–256. doi:10.1016/0006-8993(89)91072-x. PMID 2804634.
- ^ a b Moratalla R, Khairnar A, Simola N, Granado N, García-Montes JR, Porceddu PF, Tizabi Y, Costa G, Morelli M (August 2017). "Amphetamine-related drugs neurotoxicity in humans and in experimental animals: Main mechanisms". Prog Neurobiol. 155: 149–170. doi:10.1016/j.pneurobio.2015.09.011. hdl:10261/156486. PMID 26455459.
MDMA dose-dependently increases DA release and production of hydroxyl radicals in the mouse striatum (Górska et al., 2014; Górska and Gołembiowska, 2014). ROS, together with reactive nitrogen species (RNS), and the formation of neurotoxic MDMA metabolites, may all contribute to neurotoxicity by MDMA (Puerta et al., 2010; Green et al., 2003). Moreover, DA and 5-HT are metabolized by MAO-B, inducing the formation of superoxide (O2-) and hydrogen peroxide (H2O2) (Capela et al., 2009). This suggests that MAO-B is involved in the neurotoxic effects of MDMA, a hypothesis which is further substantiated by the finding that the MAO-B inhibitor selegiline prevents serotonergic neurotoxicity in MDMA-treated rats (Sprague and Nichols, 1995; Alves et al., 2007).
- ^ a b Puerta E, Aguirre N (July 5, 2011). "Methylenedioxymethamphetamine (MDMA, 'Ecstasy'): Neurodegeneration versus Neuromodulation". Pharmaceuticals. 4 (7): 992–1018. doi:10.3390/ph4070992. ISSN 1424-8247. PMC 4058674.
It had been proposed that one source of free radicals observed after MDMA administration is the metabolism of dopamine by monoamine oxidase-B (MAO-B) to produce H2O2 that is reduced by iron to produce hydroxyl radicals and a subsequent terminal degeneration [140-143]. This suggestion is supported by the fact that MDMA elicits dopamine release [144] and the dopamine precursor L-3,4-dihydroxyphenylalanine (DOPA) exacerbates MDMA-induced 5-HT depletions in the rat [145]. Conversely, inhibition of dopamine synthesis and blockade of its uptake by mazindol attenuates MDMA-induced long-term depletion of brain 5-HT content [146,147]. Moreover, inhibition of MAO-B, either by pharmacological agents (L-deprenyl also termed selegiline) or antisense oligonucleotide targeted at MAO-B, was shown to be protective [140,141,148,149].
- ^ Sprague JE, Nichols DE (April 1995). "Inhibition of MAO-B protects against MDMA-induced neurotoxicity in the striatum". Psychopharmacology. 118 (3): 357–359. doi:10.1007/BF02245967. PMID 7542394.
- ^ Sprague JE, Nichols DE (May 1995). "The monoamine oxidase-B inhibitor L-deprenyl protects against 3,4-methylenedioxymethamphetamine-induced lipid peroxidation and long-term serotonergic deficits". J Pharmacol Exp Ther. 273 (2): 667–673. PMID 7538579.
- ^ Alves E, Summavielle T, Alves CJ, Gomes-da-Silva J, Barata JC, Fernandes E, Bastos Mde L, Tavares MA, Carvalho F (September 2007). "Monoamine oxidase-B mediates ecstasy-induced neurotoxic effects to adolescent rat brain mitochondria". J Neurosci. 27 (38): 10203–10210. doi:10.1523/JNEUROSCI.2645-07.2007. PMC 6672671. PMID 17881526.
- ^ Corkery JM, Elliott S, Schifano F, Corazza O, Ghodse AH (July 2013). "MDAI (5,6-methylenedioxy-2-aminoindane; 6,7-dihydro-5H-cyclopenta[f][1,3]benzodioxol-6-amine; 'sparkle'; 'mindy') toxicity: a brief overview and update". Hum Psychopharmacol. 28 (4): 345–355. doi:10.1002/hup.2298. PMID 23881883.
There is no information on MDAI's long-term effects. Studies on rats suggest that the substance does not cause neurotoxicity (Nichols et al., 1990; Nichols and Oberlender, 1991). However, when used with dopamine-releasing agents, it contributes to toxicity in rats (Johnson et al., 1991c). Attempts by Sprague et al. (1996) to potentiate the selective serotonergic neurotoxicity of MDAI with either a non-specific dose of the MAO-B inhibitor L-deprenyl (10 mg/kg) or the MAO-A inhibitor chlorgyline (2 mg/kg) were unsuccessful. This contrasts with the findings of L-deprenyl potentiating non-neurotoxic doses of PCA (pchloroamphetamine) (Benmansour and Brunswick, 1994). The findings of Sprague et al. (1996) suggest that MDAI is a safe substance in this regard. However, these assumptions about the apparently benign activity of MDAI at recreational levels are based on animal experiments and in vitro cell culture work. Thus, it remains unclear whether recreational doses in man would lead to neurotoxicity (Sainsbury et al., 2011).
- ^ Johnson MP, Huang XM, Nichols DE (December 1991). "Serotonin neurotoxicity in rats after combined treatment with a dopaminergic agent followed by a nonneurotoxic 3,4-methylenedioxymethamphetamine (MDMA) analogue". Pharmacol Biochem Behav. 40 (4): 915–922. doi:10.1016/0091-3057(91)90106-c. PMID 1726189.
- ^ Halladay AK, Kirschner E, Hesse K, Fisher H, Wagner GC (November 2001). "Role of monoamine oxidase inhibition and monoamine depletion in fenfluramine-induced neurotoxicity and serotonin release". Pharmacol Toxicol. 89 (5): 237–248. doi:10.1034/j.1600-0773.2001.d01-154.x (inactive July 6, 2024). PMID 11881977.
{{cite journal}}: CS1 maint: DOI inactive as of July 2024 (link) - ^ Benmansour S, Brunswick DJ (July 1994). "The MAO-B inhibitor deprenyl, but not the MAO-A inhibitor clorgyline, potentiates the neurotoxicity of p-chloroamphetamine". Brain Res. 650 (2): 305–312. doi:10.1016/0006-8993(94)91796-5. PMID 7953696.
- ^ Sprague JE, Johnson MP, Schmidt CJ, Nichols DE (October 1996). "Studies on the mechanism of p-chloroamphetamine neurotoxicity". Biochem Pharmacol. 52 (8): 1271–1277. doi:10.1016/0006-2952(96)00482-0. PMID 8937435.
- ^ Goldberg SR, Yasar S (1997). "Methamphetamine Administration and Associated Neurotoxicity: Effects of Selegiline (l-Deprenyl)". Neurochemistry: Cellular, Molecular, and Clinical Aspects. Boston, MA: Springer US. pp. 327–330. doi:10.1007/978-1-4615-5405-9_55. ISBN 978-1-4613-7468-8.
It is possible that selegiline treatment might ameliorate the neurotoxicity seen with extended administration or self-administration of high doses of methamphetamine through it's uptake actions or other neuroprotective actions (e.g., 17, 18), although the little preclinical evidence available (19, 20) indicates no protection against methamphetamineinduced dopamine depletions.
- ^ Wan FJ, Shiah IS, Lin HC, Huang SY, Tung CS (June 2000). "Nomifensine attenuates d-amphetamine-induced dopamine terminal neurotoxicity in the striatum of rats". Chin J Physiol. 43 (2): 69–74. PMID 10994696.
- ^ Grasing K, Azevedo R, Karuppan S, Ghosh S (January 2001). "Biphasic effects of selegiline on striatal dopamine: lack of effect on methamphetamine-induced dopamine depletion". Neurochem Res. 26 (1): 65–74. doi:10.1023/a:1007632700126. PMID 11358284.
- ^ Davidson C, Chen Q, Zhang X, Xiong X, Lazarus C, Lee TH, Ellinwood EH (November 2007). "Deprenyl treatment attenuates long-term pre- and post-synaptic changes evoked by chronic methamphetamine". Eur J Pharmacol. 573 (1–3): 100–110. doi:10.1016/j.ejphar.2007.06.046. PMID 17651730.
- ^ Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remião F, Carvalho F, Bastos Mde L (August 2012). "Toxicity of amphetamines: an update". Arch Toxicol. 86 (8): 1167–231. doi:10.1007/s00204-012-0815-5. PMID 22392347.
Despite some MAOi being used by amphetamines consumers to "increase the peak", frequent fatal outcomes due to the potentiation of the pro-serotonergic effects of this association have been reported (Pilgrim et al. 2010, 2011, 2012; Vuori et al. 2003), mainly related to the consumption of amphetamines along with MAO-A inhibitors (e.g., moclobemide, clorgyline). Contrary to what is observed with MAO-A inhibitors, the selective MAO-B inhibitors (e.g., selegiline, L-deprenyl) seem to confer some protection against MDMA neurotoxicity by decreasing the depletion of 5-HT in the brain, the formation of reactive species resulting from MAO-B action on the released catecholamines, and therefore the lipid peroxidation and the oxidative damage to the mitochondria (Alves et al. 2007, 2009; Sprague and Nichols 1995).
- ^ Edinoff AN, Swinford CR, Odisho AS, Burroughs CR, Stark CW, Raslan WA, Cornett EM, Kaye AM, Kaye AD (2022). "Clinically Relevant Drug Interactions with Monoamine Oxidase Inhibitors". Health Psychol Res. 10 (4): 39576. doi:10.52965/001c.39576. PMC 9680847. PMID 36425231.
- ^ a b Braddock JA, Church DB, Robertson ID (2004). "Selegiline Treatment of Canine Pituitary-Dependent Hyperadrenocorticism" (PDF). Australian Veterinary Journal. Archived from the original (PDF) on November 29, 2010. Retrieved April 8, 2011. (PDF)
- ^ a b Eghianruwa K (2014). Essential Drug Data for Rational Therapy in Veterinary Practice. AuthorHouse. pp. 127–128. ISBN 978-1-4918-0010-2.
- ^ a b "Anipryl Tablets for Animal Use". Drugs.com. Retrieved August 31, 2017.
- ^ Lundgren B. "Canine Cognitive Dysfunction". Veterinary Partner. Retrieved April 8, 2011.
- ^ a b Riviere JE, Papich MG (2013). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. p. 530. ISBN 978-1-118-68590-7.
- ^ a b c Papich MG (2015). Saunders Handbook of Veterinary Drugs: Small and Large Animal. Elsevier Health Sciences. p. 722. ISBN 978-0-323-24485-5.
External links[edit]
- Anti-aging substances
- Antidepressants
- Antiparkinsonian agents
- Aphrodisiacs
- Attention deficit hyperactivity disorder management
- Drugs with unknown mechanisms of action
- Enantiopure drugs
- Monoamine oxidase inhibitors
- Neuroprotective agents
- Nootropics
- Norepinephrine-dopamine releasing agents
- Phenethylamines
- Prodrugs
- Propargyl compounds
- Sigma receptor ligands
- Stimulants
- Substituted amphetamines
- TAAR1 agonists
- World Anti-Doping Agency prohibited substances
