Talk:Orbital hybridisation
| This is the talk page for discussing improvements to the Orbital hybridisation article. This is not a forum for general discussion of the article's subject. |
Article policies
|
| Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
| This article is written in British English, which has its own spelling conventions (colour, travelled, centre, defence, artefact, analyse) and some terms that are used in it may be different or absent from other varieties of English. According to the relevant style guide, this should not be changed without broad consensus. |
| This It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
General Discussion
[edit]Over the next several days/several edits I'm going to try and overhaul this article and make it more comprehensible. I've got it on watch, so any sugestions along the way are welcome. — Preceding unsigned comment added by EagleFalconn (talk • contribs)
- I think it could have more cross-referencing for the introduction, and a more focused body. There's perhaps just two much writing that's introductory, and it makes it a bigger read than necessary. I think another article shoud introduce the theory of atomic orbitals, in terms of the solutions to the Schrodinger equation for electrons. I'm going to see if something like that exists. Then, the hybridized orbitals can be introduced as a mixing of the atomic orbitals, as a linear combination of basis (linear algebra)states. This would improve the understanding that hybridized orbitals are the result of multiple basis states existing in superposition, with a significant energetic stabilization due to resonance in hybridized orbitals compared to atomic ones. So I think this could use more work (yes, I'll do some!) rmbh 07:08, Nov 21, 2004 (UTC)
- Oh wow! The molecular orbital page builds all the way to hybridization, and it doesn't link to this page! There's been a lot of duplicated effort here...it's really the molecular geometry concepts that are unique to this article. The specialized hybridized orbital pages should probably be incorporated here, instead of the bulk of it, which is better explained in the molecular orbital page. rmbh 07:34, Nov 21, 2004 (UTC)
- I was thinking about it over the weekend, and I was considering expanding on the bottom section and discussing in more detail the various forms of hybridization. I realized it would be fairly redundant , though I don't believe a page exists for any hybrids except sp², which right below is indicated as needing work as well. But you're right, the molecular orbital page certainly does do much of the pre-hybrid explanation work. I feel like it is explained a little better with the discussion of methane, though everything prior to where the stress exists that orbitals are models could be considered unnecessary. I think that the explanation in terms of molecular orbitals and in terms of atomic orbitals are unique and each contribute in separate ways, so there may be merits for leaving it there. Also, you have to consider: More people are familiar with atomic orbitals than molecular. I'm something of a Wikipedia noob, so perhaps this is a dumb question: Is the goal of Wikipedia with this page to educate someone who has the majority of the background knowledge for this article, or to try and relate it to someone who might not be all the way there? --EagleFalconn 15:57, 22 Nov 2004
- You raise a dead-on point. I think that the Wicki approach constantly raises this issue: as a piece of unified writing, introductory material is required, but as a Wicki, introductory material is largely redundant, and clutter-promoting. After perusing Wickipedia:Forum for Encyclopedia Standards, I expect that the latter view dominates amoung Wickipedians. An obvious exception is large overview articles, like 'Canada' which may contain 'Canadian Bacon'. A subject as broad as 'quantum chemistry' probably has no such centralized article for exposition; perhaps a history article would suffice. In general, articles should be both accessible and of sufficient depth to be useful. I mean, everyone knows, Britannica will tell you more than World Book, which makes it better for researchers than for elementary schools. I think only time will tell, but there's a lot of expertise out there, and Wickipedia seems to draw unprecedented attention, so it's possible that the two approaches are not mutually exclusive.
- I agree that the entries for the popular handles for hybrid orbitals should be kept. I agree that hybridization should be kept as well, but most of the page should be moved to molecular orbitals. There is plenty of complementary material, and we could try to edit the bulk of the article starting with that configuration.
- Hybridization is, specifically, the 'mixing' of atomic orbitals into molecular ones. The current hybridization article does a good job of explaining that molecular symmetry is difficult to reconcile with atomic orbitals. I would add (I propose to add!) that the quantum indistinguishability of the groups surrounding the carbon atoms are the source of MO symmetries. To satisfy these necessary symmetries (described by group operations, connecting to group theory), the atomic orbitals are hybridized. The actual hybridization is represented by a linear combination, or superposition of atomic orbitals.
- So I like the idea of the hybridization article not defining 'orbital'. By ignoring the actual wave-equation, the article can discuss the geometric implications of hybridizations, which is arguably the 'champion concept' (go Pauling!), without considering things like antibonding orbitals, or the exlusion principle. Definitely keep the methane, although it stinks! ;) rmbh 02:22, Nov 23, 2004 (UTC)
- I think the problem with merging the two articles is the approach. Molecular orbitals certainly do lead right into hybridization, but from a very different angle than the one the article currently takes. Merging the introductary material also poses a problem. I'm not sure that they really are cohesive and belong in the same article. We could create two separate articles, "Molecular Orbital approach to Hybridization" and "Atomic Orbital Approach to Hybridization" but that seems unnecessarily extensive. Perhaps the blatantly redundant information could be removed, and a link at the top of the article to molecular orbital saying "A different approach to hybridization can be gleaned from the article on molecular orbitals in lieu of the explanation in terms of atomic orbitals given here." EagleFalconn 03:16, 23 Nov 2004 (UTC)
I think that since we've established that the page needs some work, but is for the most part better than it was, I'm going to remove the attention tag. EagleFalconn 01:46, 6 Dec 2004 (UTC)
Ok, I'm way late into this conversation, and I came to discuss my other topic below, but I'll leave my thoughts on this issue anyway. The current thinking is that the best articles are written in Wikipedia:Summary style, where the main article has the overview and relevant details to fit in about 30k of text, and the various subsections for more detailed topics are summaries of more detailed daughter articles. Then the lead section for the main article is a overview summary designed to ease a reader into the whole topic. Using good summary style has a number of advantages, including avoiding having duplicate articles covering related material in different ways. Ideally all of the important ways of looking at the topic should be summarized in the main article, and the daughter articles would cover the details. Yes the summary subsections cover the same material as the daughter articles, but that redundancy is inevitable and even good. It allows a topic to be covered on many levels, from the superficial to the gory details, and satisfy everyone from the novice to the expert. It's not easy of course, but who said any great article was. Suffice it to say that there is a lot of consensus for this way of organizing the best articles. Thanks - Taxman Talk 23:59, Jun 9, 2005 (UTC)
Revision?
[edit]A problem with hybridization is that it is really only used in elementary texts, and this leads to confusion. Thus
- hybridization has nothing to do with molecular orbitals, but is purely to reconcile the directions of atomic orbitals with molecular shape when used in simple valence bond theory.
- hybridization using d orbitals is totally discredited, although inertia keeps it in the text books!
clearly the guys who are referring to molecular orbitals and hybridization have something in mind, but as the terms are properly - and widely - used, they are not related at all.
.. more thoughts later...--Ian 12:08, 31 Jan 2005 (UTC)
PS: should we settle on hybridization or hybridisation? The former is used in most books I've seen (both US and UK)....
I'm going to have to disagree Ian...I perform computational chemistry research at Indiana State University and orbital hybridization is most definetely a theory that is still used. For example, the NBO package which uses the schrodinger equation to evaluate the bonding orbitals of a molecule is cutting edge stuff. It displays bonds in terms of hybrids. Furthermore, d orbital hybrids are not debunked in any way...could you cite some sources as to your opinions because those are both certainly news to me. Also, since this is the English Wikipedia, I would assume that it is US/UK centric, and should be spelled with a Z. EagleFalconn 02:39, 1 Feb 2005 (UTC)
I too am in computational chemistry research, so maybe we should work something out wihtout clogging up the main pages. I've put some sources and comments on your talk page. Suffice to say here that NBO is an interpretation of results that come from wavefunctions, cast especially to bring in hybridization - it certainly isn't there in the first place! I too reckon Z rather than S - but the article doesn't! --Ian 15:19, 1 Feb 2005 (UTC)
- Well that is related to what I wanted to ask. I'm certainly not on the level of you guys, but I have skimmed Pauling's book and some other Quantum Chemistry books. Based on what I know, the article seems very wrong in this paragraph:
- The answer is the theory of hybridisation. At this time it is important to note that orbitals are, and always will be, a model. They are not real. They are derived from specialised solutions of the Schrödinger equation and therefore to hybridise, to mix an orbital, is simply to change the mathematical function governing where that electron should be. It is not altering the structure of the atom in any way.
- Yes they are a model, and have to be, since we can't see the electrons directly. But doesn't this miss the point that the hybridization model is very very satisfactory and predictive for how the structure and reactivity of carbon compounds? Isn't there evidence that the hybridization model is the "right" way to solve the equations for the probabilities of where the electrons are? In other words, isn't it well known that for methane for example, the hybridization model fits very well with what we know to be the forces of the electrons and bonding and the resulting location of the hydrogen atoms? Stating that we are "changing the mathematical function" and "altering the structure" seems to be ignoring that fact. We solve the Schrödinger equation in that way because we believe it fits with observations. And of course it's not altering the structure of the atom. The structure is what it is, we just believe the hybridization modle correctly describes the structure of the molecule. - Taxman Talk 23:59, Jun 9, 2005 (UTC)
- Hmm...sort of jumping in here too, so apologies if I've misinterpreted the question. The concept of hybridization in my opinion is exceptionally good for organic chemistry. However, for other other areas, say transition metal chemistry, hybridization isn't nearly so good (in fact it is rather poor). You'd need a lot more sophisticated concepts to deal with it. Nevertheless, hybridization can be cast into a set of heuristics that are very useful for chemistry. I'm not sure what the paragraph refers to when it says "altering the structure...", but I think this has to do with the assumption that each atom (or molecule for that matter) can be approximated as a linear combination of atomic orbitals (LCAO). This explains why hybridization is good for organic chemistry. One is only dealing with "light atoms", as opposed to "heavy atoms" where this assumption does not hold anymore. Furthermore, simple schemes of hybridiziation assume we are using atomic orbitals from hydrogen atoms, since this is the only atom for which we can actually compute analytically their wavefunctions! So in fact, a number of simplification methods are being used here, and they are at best an extremely good heuristic. Hybridization does not have nearly as much predictive power as one would like unfortunately. --HappyCamper 15:24, 30 July 2005 (UTC)
- No, I think you've answered it pretty well, but then it seems that the article is in error in the points I pointed out, if only because it does not make the distinction you make between large and small. I don't think I have the expertise to correct that paragraph and not add worse errors. If you could, that would be great. - Taxman Talk 15:48, July 30, 2005 (UTC)
- Hmm...I made some changes to the page, but later I might change it again when I get the chance. I think I introduced quite a number of loopholes into the article now. At least it was a good attempt at fixing it up! I just realized that these chemistry related pages need a lot of work. There is so much content that is missing, fundamental concepts which really should be part of this encyclopedia, I think. Can't wait until the end of August when I've got more time to play on Wikipedia... --HappyCamper 16:56, 30 July 2005 (UTC)
- *gasp* Wikipedia doesn't even have an article on LCAO?! Too many articles to write, too little time...no wonder why we need "Wikipediholics Anonymous"! --HappyCamper 15:24, 30 July 2005 (UTC)
- Actually, we have an article at Linear combination of atomic orbitals molecular orbital method, so I redirected LCAO there. I hope that is correct. - Taxman Talk 16:37, August 1, 2005 (UTC)
- *gasp* Wikipedia doesn't even have an article on LCAO?! Too many articles to write, too little time...no wonder why we need "Wikipediholics Anonymous"! --HappyCamper 15:24, 30 July 2005 (UTC)
- Well, it's not quite precise, but I guess it will do. I was thinking that LCAO should only talk about what LCAOs actually are, instead of describing an application of them. When I get the time I'll fix it up. This is too interesting...the reference text in that article I actually have!! --HappyCamper 17:21, 1 August 2005 (UTC)
- Another issue I had with this article is the hybridization for hypervalent molecules. The theory that d orbitals play a role in hybrid molecular orbitals is obsolete due to the high energy difference between d and p orbitals. A new theory states that a molecule like Phosphorous V Chloride has sp2 hybridization (with one shared resonance p orbital) and Sulfur VI Fluoride has a sp hybridization (with two shared resonance p orbitals). This is still debated among chemists but the new theory is more plausible in light of energy differences. --Falconer Talk 01:23, November 8, 2005 (UTC)
Spelling
[edit]I prefer using "hybridization" rather than "hybridisation". --HappyCamper 15:25, 30 July 2005 (UTC)
- Well in general if it is a difference between the British English and the American, policy is not to change from one to another once it is set. But from the above conversations it looks like it is spelled with a 'z' in both, so why is it 's' at all? - Taxman Talk 15:48, July 30, 2005 (UTC)
- Yeah, I know...odd isn't it? Well, I habitually spell with "z", but if the article insists on "s", then hopefully my nimble fingers will remember that when I edit :) --HappyCamper 16:36, 30 July 2005 (UTC)
- But it isn't. Hybridisation and hybridise are just like any other words ending in -isation/-ise in British English. Just like realise/realisation or organise/organisation. So they aren't exceptions in British English at all. What I think might have been meant above is that such words are on the rare occasion spelt with a zed in British English because the Oxford English Dictionary prefers -ize/-ization on some etymological grounds, but this is ever more seldom encountered in Britain (and perhaps it is even rarer in countries such as Australia and New Zealand). All British newspapers to the best of my knowledge, for example, use s forms; and in fact, most Britons would find it oddly American to use -ize, even if it was in the past used by perfectly good English writers. See this and this Wikipedia page for some detail.
- My books, both textbooks for school/sixth-form as well as the ones used at university, certainly speak of hybridisation (except for the odd one which wasn't written in Britain!). It's definitely and by far the commonest spelling in Britain, so I reckon this article should stay where it is. 213.143.80.81 05:30, 2 August 2005 (UTC)
- Ah very well then. In that case, the policy is clear: we leave the spelling where it is. I just misinterpreted what was above I guess. - Taxman Talk 05:43, August 2, 2005 (UTC)
- I don't believe hybridise is a word in UK English or US English. I am unable to find a real dictionary that has a definition for Hybridise, though I don't have a subscription to OED to check it for sure, I have checked other dictionaries that usually have both spellings and they all have Hybridize but no "ise" version. - Pete Davis 14:12, July 18, 2006 (UTC)
- Ah very well then. In that case, the policy is clear: we leave the spelling where it is. I just misinterpreted what was above I guess. - Taxman Talk 05:43, August 2, 2005 (UTC)
(moved here from main page Dirk Beetstra T C 08:47, 12 December 2006 (UTC)): The word hybridisation is spelled wrong many times in this article and should be replaced with the correct spelling hybridization.—Preceding unsigned comment added by 70.243.254.146 (talk • contribs)
- I am sorry, the original page was written in UK EN, hence name 'orbital hybridisation', and the use of the UK spelling 'hybridisation' throughout the article. People searching for 'orbital hybridization' will be redirected to this page, so they still can find it. Hope this explains. --Dirk Beetstra T C 08:47, 12 December 2006 (UTC)
Hybridise certainly is an English word. Being in Australia I only have access to the Macquarie Dictionary, but the entry there is:
- --verb (t) 1. to cause to produce hybrids; cross. 2. to form in a hybrid manner.
- --verb (i) 3. to cause the production of hybrids by crossing different species, etc.
- --hybridisable, adjective
This of course is where the word in chemistry came from. --Bduke 22:26, 30 November 2007 (UTC)
I'm in the US, so I'm happy with the zed, I mean the zee. However, regardless of one's leaning on this spelling issue, it seems reasonable to have uniformity within the page. This article apparently was started with hybridisation, so that's what I've changed everything to. -- Astrochemist 02:26, 29 April 2007 (UTC)
- See WP:ENGVAR for Wikipedia's guideline about this spelling issue. DMacks 01:41, 30 April 2007 (UTC)
One guideline at WP:ENGVAR says "Articles should use the same spelling system and grammatical conventions throughout." Since this particular article was started with British English, are there other words in it that should be changed too? Someone from the UK, Canada, and so on could probably spot them faster then me. -- Astrochemist 02:31, 30 April 2007 (UTC)
Since Wikipedia and Wikimedia are both American, the articles should be spelled in American English. You wouldn't find colour in an American book, and you wouldn't find color in an English text. The Brits have enough dictionaries/encyclopedias, wikipedia is ours! Also, hybridisation looks really ugly. —Preceding unsigned comment added by 152.3.152.242 (talk)
- I don't think a wrong premise (WP is an international thing) and your bias about how something looks are good reasons to go against official WP guidelines that represent a consensus opinion of the editors. DMacks 17:33, 30 November 2007 (UTC)
- Well WP guidelines state that national ties allow a change from one spelling to another- Linus Pauling, the man who discovered orbital hybridization was American, and wrote his original publications in American English, calling it Hybridization himself. Too be more accurate, WP should also call it hybridization. —Preceding unsigned comment added by 152.3.78.56 (talk) 01:33, 4 December 2007 (UTC)
- It is not an "American" concept though. DMacks (talk) 02:50, 7 December 2007 (UTC)
- Since it was discovered by an American, I think it's better to honor the original spelling.128.2.247.27 (talk) 20:05, 2 September 2009 (UTC)
- It is not an "American" concept though. DMacks (talk) 02:50, 7 December 2007 (UTC)
- Well WP guidelines state that national ties allow a change from one spelling to another- Linus Pauling, the man who discovered orbital hybridization was American, and wrote his original publications in American English, calling it Hybridization himself. Too be more accurate, WP should also call it hybridization. —Preceding unsigned comment added by 152.3.78.56 (talk) 01:33, 4 December 2007 (UTC)
I changed it Look here "American spelling accepts only -ize endings in most cases, such as organize, recognize, and realize.[48] British usage accepts both -ize and the more French-looking -ise " It needs to be ize...if we are looking for a generally more accepted term, ize works for everyone. Bozgoalie (talk) 22:37, 16 November 2009 (UTC)
- I appreciate your WP:BOLD change, but need to get some current WP:CONSENSUS that your way is better than inertia (which is a valid reason to keep it with "s"). DMacks (talk) 22:47, 16 November 2009 (UTC)
- Hybrid is Latin, not Greek, so it should be "hybridise". Also, per WP:ENGVAR, the article should continue with the title it's got. "Hybridize" is just weird. Jheald (talk) 00:53, 17 November 2009 (UTC)
Diagram
[edit]In the methane hybridisation picture towards the bottom of the article, the nodes appear to coincide with the nucleus which is, I am assured, incorrect. The nucleus should lie a little way into the minor lobe. Mullet 13:24, 27 October 2005 (UTC)
Carbon bonds
[edit]I have a question on this quote from the article: "Carbon will never form any less than four bonds unless it is given no other choice, which seldom occurs."
I have been taught that carbon ALWAYS forms four bonds, now this may be because I'm not a chem major yet and it's somethign to be viewed later, but could anyone verify and confirm wether the quote is accurate? The PA 01:40, 8 December 2005 (UTC)
- Always is a dangerous word. Carbon doesn't always form four bonds, but if it can its significantly more stable for it to do, and if the activation energy is low enough and if the process is exothermic enough (ie, enough to prevent simple reconversion to some other bonding state) it will. There are exceptions. Lone carbon atoms, certain reaction intermediates, carbocations. EagleFalconn 07:25, 6 January 2006 (UTC)
Limited usage to certain groups?
[edit]I removed this from the introductory paragraph:
- ... made of atoms of the groups 2,3,4 of the periodic families of elements. On the other hand, groups 1,5,6 and 7 in the periodic table do not hybridise, i.e. this approach is in that case not useful.
That is in contradiction with the VSEPR theory, in which hybridization of atoms of groups 5 and 6 are useful. Please do some research before putting it back in. -- Felix Wan 01:21, 3 February 2006 (UTC)
- It's been tagged as needing verification and sources; hopefully the original main authors will provide the sources, but it might need to be combed through anyway. --AySz88^-^ 19:24, 4 February 2006 (UTC)
- Is this where the dispute is? To fix this article, I think it is important to nail what exactly it should be talking about. I think it would be more valuable to focus on the heurstic aspects of the theory first, and if we have time, add some results about ab initio calculations. --HappyCamper 04:12, 13 May 2006 (UTC)
Hmm....
[edit]Well, well, well...I think I might be bold on this article.
At minimum, I'd like to add these to the bottom. (And they are redlinks too!)--HappyCamper 14:37, 13 May 2006 (UTC)
- Dear HappyCamper, I would like to know what you mean with the remarks in this section. The problem is, I have a similar feeling about this article .. when reading this I get the feeling that people are led to believe that hybridisation really exists. Now I do understand that it is difficult to understand the bonding in methane, when the 3 p-orbitals are pointing in 6 directions, while there are (in general) only 4 atoms around C. It is a very useful concept, but not 'the truth' (if that exists, orbitals are already a concept .. etc. etc.)
- By the way, since this evening, Tanabe-Sugano diagrams is not a redlink anymore, though the page contains hardly any info, I just hope that some spectroscopist will really kickstart the page (not my specialisation), it is IMHO really missing. --Dirk Beetstra 20:32, 22 May 2006 (UTC)
Hybridization theory has been superceded by MO theory?
[edit]Hi Smokefoot, Hybridization theory superseded by MO theory? I suggest that the orbital hybridization page starts with an explanation of the concept followed by a criticism section and not the other way around. I notice that the MO theory page completely lacks elemental MO diagrams let alone that of methane. I have seen this before in the Banana bond page where one editor was so certain that Banana bonds were made redundant by Walsh orbitals that he advised wiki readers to forget about banana bonds altogether. The Walsh orbital page has yet to be written.... My suggestions: we should try to find a methane photoelectron pic like this one here , explain how it is possible to fix orbital hybridization theory in order to accommodate this uncomfortable truth and move criticism part to the bottom, and also start working on a MO diagram page V8rik 15:46, 16 September 2006 (UTC)
- Hi V8rik, I figured that you would have useful suggestions. My goal was a short "disclaimer" early in the article to prevent impressionable readers believing hybrid orbitals (not that I so much of a theorist to strongly complain, but hybrids have just disappeared from modern textbooks). But I will follow your recommendation to move the section lower down. About getting a photo-electron spectrum on the MO or methane page, that would be desirable, but I dont know where to look for a copyright-ok spectrum. I have ppt slides of qualitative MO's for methane and one of these days I will figure out how to load graphics on WE. --Smokefoot 16:41, 16 September 2006 (UTC)
- Hi Smokefoot, Thanks for your consideration. Finding spectra for insertion into Wiki is always a problem but I will add some external links to spectra later this evening and some related text. I will also will be happy to collaborate on a MO diagram article, I really think Wiki needs one V8rik 16:50, 16 September 2006 (UTC)
(If you can tell me how to better save this ChemDraw, that would be appreciated, it looks grainy - I just saved ChemDraw as .png). Is this the kind of graphic that you were thinking of? I also have a drawing (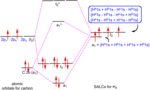 ) somewhere in wikispace but I dont know how to work with it - it does not seem to load. Possibly too big. In any case, this is the kind of stuff I have for a future MO diagram article.--Smokefoot 18:40, 16 September 2006 (UTC)
) somewhere in wikispace but I dont know how to work with it - it does not seem to load. Possibly too big. In any case, this is the kind of stuff I have for a future MO diagram article.--Smokefoot 18:40, 16 September 2006 (UTC)
- that is already more advanced than what I had in mind: something like Link. there exists a nice but peculiar way to save images from chemdraw, summarised here: [1], it will make the images very sharp. V8rik 20:09, 16 September 2006 (UTC)
- Hi Smokefoot, I have researched some recent general organic chemistry textbooks and I must conclude that hybridization theory is very much alive as far as organic chemists are concerned. I took the liberty to rephrase the paragraph header and added my findings. V8rik 22:05, 29 September 2006 (UTC)
- V8rik yes, the organickers love hybridization. And its seems unlikely that this terminology will slip soon. --Smokefoot 22:21, 29 September 2006 (UTC)
Well, I am one of those organickers. I have read the comments on Orbital hybridization and Revision. What I do not find are arguments that use MO theory to explain the structure of methane, ethylene, acetylene, etc. These always seem to use hybridization theory. If hybridization theory is incorrect (as a model), then explain how or why MO theory is superior for the examples that seem to prefer hybridization theory. (Or perhaps I don't have a correct understanding of MO theory.)
I understand how Pauling would have adopted hybridization to explain the bonds of methane. Pauling was more pragmatic than theoretical. Without going into detail, I think there should be doubt about interpretation of black body radiation as solely electron energy levels. Doing so indicates electrons fall to higher and higher energy levels to explain the smaller energy gaps. The emission to the lowest energy level has the highest energy gap. While I agree the emission spectra follow a quantum energy pattern (Balmer and Rydberg), I don't agree that Bohr has PROVEN these energy difference do correspond with electron transitions. (I am borrowing from Gilbert Lewis's comment in his 1914 JACS paper.)
Never the less, if the point being made is that MO theory has superseded hybridization theory, then a greater explanation of why this should be the case should be made. I am not an expert, but if Pauling (and his followers) has erred, explain how. The photoelectron spectrum of methane is data. It shows there are different energy levels. How does this data prove MO theory as the sole correct explanation? How does MO theory not imply that one hydrogen is different than the other three in methane?
This is what I would like to see. If MO theory is superior for inorganic compounds and perhaps hybridization is better for small atoms, then give examples of data that are analyzed by each and a brief explanation. The point of these examples wouldn't be to answer which is superior, but rather to explain what each is and how (or why) they are best used. Petedskier (talk) 19:46, 30 December 2011 (UTC)
- agree , there is a contradiction when you have 100 people say forget about hybridization and stick to MO theory when it comes to methane when at the same time there is no information at all on methane/MO. The hybrid model wins by popular vote V8rik (talk) 20:28, 30 December 2011 (UTC)
Valence shell electron-pair repulsion (VSEPR) theory
[edit]The statement "hybridisation is an integral part of valence bond theory and the valence shell electron-pair repulsion (VSEPR) theory" is in fact incorrect in the latter part as the original authjor of this VSEPR approach claims that there are no orbitals involved in the theory. I do not have the references given to hand but I have no doubt they support the statement. They are however wrong as are so many others. Of course you might argue that VSEPR is incoherent if it it does not implicitly include orbitals of some kind, but that is WP:OR. Gillespie would not agree. Because this is controversal I bring it here rather than just delete it. --Bduke 02:45, 14 December 2006 (UTC)
- I've deleted the VSEPR claim. VSEPR doesn't require hybridization, it's just a qualitative model that tries to place the electron pairs as far apart as possible (like charges on a sphere). The fact that the geometries predicted agree with those corresponding to spmdn hybridizations is a coincidence that was unfortunately taken too far by general chemistry textbooks for some time. Now that there's consensus that involving the d orbitals is not correct, most modern textbooks talk about VSEPR without involving hybridization.--Itub 08:53, 9 May 2007 (UTC)
- Taxman reverted my deletion, saying "the VSEPR part appears to be part of a referenced fact. If you have beeter sources, adjust it of course. Either way the article should link and discuss the relation to VSEPR". I checked the sources, and they make no such claim. I'm sure you could find some old general chemistry textbook that wrongly makes such claim, but that doesn't mean we need to keep propagating such nonsense. I've cited a recent article by R. J. Gillespie, the author of the VSEPR theory, which clearly says that VSEPR is completely unrelated to orbitals and to valence bond theory. --Itub 16:11, 9 May 2007 (UTC)
- Right on the nail. Gillepie says the same thing in more detail in a Chemical Society Review. --Bduke 02:08, 10 May 2007 (UTC)
- Yes, for small molecules the "flow of information" is usually (1) start with a molecular formula, (2) draw a Lewis-dot structure, (3) apply VSEPR to predict the electron-pair and molecular geometries, and (4) apply VB to get approximate compositions of hybrid orbitals. If your main interest is structure (geometry) you stop after step (3). In other words, you apply VSEPR first, get an answer, and don't need to worry about VB to explain (check, etc.) the VSEPR prediction. -- Astrochemist 02:50, 10 May 2007 (UTC)
Bond angles and hydridisation ratios
[edit]For experimentalists, I think that the most-common approach to VB theory is determine a structure (spectroscopic, diffraction, etc.) without reference to orbital hybridisation. As this article's "Hybridisation and molecule shape" section describes, mathematical relations exist to connect bond angles and hybridisation ratios, so if one can get (by VSEPR or measurement) a bond angle then a hybridisation ratio follows. I'm not sure how often the reverse is be done, getting a bond angle (structure) from a p-to-s ratio. There certainly are cases in EPR/ESR when isotropic and anisotropic hyperfine coupling constants can give p-to-s ratios, which in turn are converted into bond angles. (The Wikipedia EPR/ESR article is poor, so I'm not citing it. There's almost nothing there about hyperfine coupling of any type.) -- Astrochemist 02:50, 10 May 2007 (UTC)
- There is a force field called VALBOND that uses hybridizations to compute the angular bending energy (and therefore the structure). The hybridizations themselves come from empirical formulas that are parameterized so that certain elements have a higher preference toward p character than others In VALBOND the possible hybrids are a continuum, including things such as sp2.81, which can lead to equilibrium angles different from the "textbook cases" of 109.47, 120, and 180 deg. --Itub 05:33, 10 May 2007 (UTC)
Redundant sp2 bond article
[edit](copied over from Talk:Sp² bond; please continue conversation here)
This article (sp2 bond) doesn't say anything that's not already in orbital hybridization or chemical bond, and "sp2 bond" is not a common term. Actually, I'd say that the term doesn't make much sense, since the hybridization is based on the atom and not on the bond. What is more common is to say "a bond involving an sp2 atom", or an sp2-sp2 bond, (or sp2-sp3, etc.). I propose deleting this article, maybe leaving a redirect to orbital hybridization. Itub 23:10, 27 December 2006 (UTC)
- I agree. Put merge tags on the two articles to draw attention to your suggestion. --Bduke 23:20, 27 December 2006 (UTC)
- support merge V8rik 20:31, 29 December 2006 (UTC)
- support merge --Dirk Beetstra T C 13:18, 6 January 2007 (UTC)
PCl5: why sp3d but s2p3?
[edit]3plx help!!!!
In PCl5, why can't the 4s orbitals be used in the "hybirdization"? Why is the hybridization of P in the compound as sp3d, but not s2p3( using a 3s, a 4s, 3 3p orbitals)?
What is mean by the statement: there is a conflict between 3s and 4s in the hybridization?
thx
Retrieved from "http://en.wikipedia.org/wiki/Talk:Trigonal_bipyramid_molecular_geometry"
58.152.216.169 12:05, 28 February 2007 (UTC)
Where is the statement that there is a conflict between 3s and 4s? The idea that you need sp3d to explain PCl5 is out-dated and is not needed. In fact there is evidence that the d orbitals are not involved in the bonding to anything like the extent suggested by sp3d - i.e. equal s and d involvement. Molecular orbital theory can explain the bonding without using d orbitals, but a very small d involvement does improve the picture. --Bduke 22:07, 28 February 2007 (UTC)
Controversy section
[edit]The view that the d orbitals contract on the central atom when the other atoms are highly electronegative was studied over many years by David Craig but never really established. The view that the d orbitals are not involved in bonding in these molecules is now well established due in part to the work of Eric Magnussen. Most General Chemistry text are beginning to remove the sp3d2 etc hybridisation discussions. I'm just about to leave for a few days and will be on wikibreak. I'll try to look at this when I return and dig up the references. --Bduke 22:14, 30 April 2007 (UTC)
Something in the second paragraph of that section doesn't seem right. The objection to s-p-d hybridization schemes seems to be based on the relative differences in size among those three types of atomic orbitals. There is then a comment about d orbitals contracting so that they are closer to the s and p types, in radial distance. However, won't this same formal-charge argument also apply to the s and p orbitals, contracting them beyond the size needed for hybrids to form with d orbitals? None of this is obvious to me, one way or another, except that the contraction argument sounds more like an after-the-fact rationalization than an a priori explanation. No, I don't have an answer and I'm not arguing one way or the other. Perhaps someone could add references for this section of the article. -- Astrochemist 00:48, 1 May 2007 (UTC)
Sp orbital?
[edit]How about an image of an sp hybrid orbital? It looks essentially like a p orbital, but it's different. 168.122.108.87 (talk) 20:19, 18 September 2008 (UTC)
- An sp hybrid doesn't look much like a p orbital- one lobe is far, far larger than the other. Two sp hybrids are orthogonal to each other at right angles, so the equivalent picture would look like two big lobes at a right angle, with tiny lobes across from each. --Noren (talk) 17:14, 14 November 2009 (UTC)
Water example
[edit]Water is an unfortunate choice of an example for this article, since water isn't actually an sp3 hybrid. This can be (and indeed has been) easily proven with XPS spectroscopy. The fact that its geometry happens to be close to what would be predicted by an sp3 hybrid is merely a coincidence.
- It's not coincidence. The valence s and three p orbitals of the oxygen are all occupied, and there is hybridization between them. The counterintuitive part that you're missing is that lone pairs and the bonding orbitals are not equivalent- proportionally more of the lower energy s orbital is used by the lone pair than by the bonding orbitals. See Bent's rule, which is tragically a bit of a stub at the moment.--Noren (talk) 17:19, 14 November 2009 (UTC)
Why not do a N central atom?
[edit]Just using carbons is a cop out. —Preceding unsigned comment added by Inthemtns (talk • contribs) 19:20, 16 October 2009 (UTC)
citation needed in sp3 section
[edit]I have added a "citation needed" tag to the sentence "The combination of these forces creates new mathematical functions known as hybridised orbitals", although it also refers to the discussion in the paragraph above leading up to that statement. I would like to know whether that explanation appears in any textbook or paper. It seems to me to be quite incorrect as it implies that hybrid orbitals are the result of physical forces. The mainstream view is that hybridisation is just a mathematical process in valence bond theory to get four distinct bonds and nothing more than that. The fact that we can quite well explain the bonding in methane without using hybrids shows that hybridisation is not a physical process. I will try to fix this but I am curious to know whether the explanation in the article has been seriously put forward. --Bduke (Discussion) 23:02, 11 January 2010 (UTC)
sp2 and ethene
[edit]Let me try to discuss the last two edits by myself and DMacks. First, "The hydrogen-carbon bonds are all of equal strength and length, which agrees with experimental data" is true but there is no basis to say it at the point where it now is as the hybridisation theory is not yet explained for ethene. It needs to be moved down to after the theory is explained. Second, The sentence above is not clear. Second, I fail to see what "(however, the π bond may or may not occur)" achieves. We are discussing ethene. The π bond does occur. This very simple idea has been badly explained in this article for two long. Another problems is that the diagram is not normally called a Kekule structure. I have had a go at rewriting this. The sentence on 2.5 hybridisation does not fit well in this section, but that is another matter. It must be very unclear to some readers. --Bduke (Discussion) 07:25, 12 February 2010 (UTC)
- My edit was merely to undo the preceding anon-IP edit, but apparently you and I had an edit-conflict involving other changes you were making at the same time. That said, the original wording was also quite confusing and in need of work and Bduke's changes certainly are an improvement. DMacks (talk) 17:39, 12 February 2010 (UTC)
- OK, that has happened to me too. I rewrote the second para and will work on the rest of the article. The lead needs work. The mention of hybridisation in MO theory, for example, is close to nonsense. Hybrids are very rarely used in MO theory and then for special reasons. It just needs removing from the lead. Others can add it later if they have sources. --Bduke (Discussion) 20:41, 12 February 2010 (UTC)
2 types of sp3d as well as absent sp2d information
[edit]There are two geometries associated with sp3d
The first is trigonal bipyramidal and can be thought of as sp3d(dZ2)
The Second is square-based pyramidal and can be thought of as sp3d(dx2-y2)
Also, There is an sp2d hybrid which corresponds to a square planar geometry.
This is shown on page 119 of "Inorganic Chemistry" 3rd edition by Catherine E. Housecroft and Alan G. Sharpe (2008)
I am unfamiliar with the controversies of the d orbital contributions so will not post revisions but discussion and investigation is needed. Perhaps these ideas have been dismissed, but if so i suggest mentioning them and explaining why they are no longer thought of that way.... but since this textbook is only 2 years old I am inclined to believe a certain portion of respectable chemists find these hybridizations convenient still (and if student's are being taught it, wikipedia should mention them).
Astote-ap (talk) 08:09, 2 March 2010 (UTC)
edit:
Upon further reading of the "controversy section" of the talk page it may be best to merge this section with that.
In support of including the d-orbital hybrids i submit that the work of PD Dr. Stefan Immel be considered. at the very least added to the list of external links on the basis that it shows pictorially and mathematically the creation of Hybrid MO's from linear combination's of AO's (http://csi.chemie.tu-darmstadt.de/ak/immel/script/redirect.cgi?filename=http://csi.chemie.tu-darmstadt.de/ak/immel/tutorials/orbitals/hybrid.html)
as noted by Dr. Immel, "hybrid orbitals are a powerful tool to describe the geometry and shape of molecules and metal complexes, yet in "real" molecules their significance may be debated. In "real" cases, someone has to refer more realistically to molecular orbitals instead. This page should give an overview on different geometries of hybrid orbitals, and the consequences for the shape of molecules."
it very well may be that the reality of d orbitals interactions are very different from Hbrid MO theory. however using Hybrid MO's with d contributions equips us with a language which can pragmatically describe the geometries of highly coordinated substances.
since Bduke is also a computational chemist, I'd like to hear in more detail his opinion before attempting to rewrite the section.
see also:
http://csi.chemie.tu-darmstadt.de/ak/immel/misc/oc-scripts/orbitals.html?id=2
Astote-ap (talk) 09:10, 2 March 2010 (UTC)
Controversy regarding d-orbital participation
[edit]This section is quite out of date. The consensus is now clear that d orbitals are not involved in bonding in molecules like SF6 any more than they are in SF4 and SF2. In all three cases there is a small and roughly identical participation of d orbitals as polarisation functions. This has been established in both MO, by Magnussen and others, and VB theory, by Cooper and others. I will try to rewrite it, but I am busy, so this is something of a reminder, but feel free to have a go. --Bduke (Discussion) 00:38, 24 April 2011 (UTC)
Removed water controversy section
[edit]I have removed the water controversy section, as unreferenced. It was filled with citation needed tags. I had never heard of this myself, so I dug through several texts, and I can find nothing to support the description written in the text. The best I can find is Bent's rule, a modification of hybridization theory which does not specifically say that (idealized) water is not sp3 hybridized, but does explain some of the deviations of real water's electronic structure from the idealized prediction of pure sp3 hybridization. The text I removed does not appear to be referenced. If someone can find solid refs, please feel free to add it back. --Jayron32 05:39, 12 May 2011 (UTC)
- I've seen the issue mentioned in several articles. You need to know where to look. Here are some references that state that oxygen in water is not sp3 hybridised:
- J. Chem. Educ. (1987) 64, 124–128: "No rabbit ears on water. The structure of the water molecule: What should we tell the students?"
- Chem. Eur. J. (2010) 16, 3663–3675: "Ligand Close Packing, Molecular Compactness, the Methyl Tilt, Molecular Conformations, and a New Model for the Anomeric Effect"
- Coord. Chem. Rev. (2008) 252, 1315–1327: "Fifty years of the VSEPR model"
- J. Chem. Phys. (2006) 124, 154307: "Bonding analysis using localized relativistic orbitals: Water, the ultrarelativistic case and the heavy homologues H2X (X = Te, Po, eka-Po)"
- We have had this discussion already some time ago on lone pair (see talk there). Picture is adequately described with cites in MO diagram. There is no reason to delete the content , the citation tags exist for a reason. V8rik (talk) 17:20, 12 May 2011 (UTC)
- That's awesome. Could you please rewrite the section, with the citations, so this embarassment does not happen any further? Thanks! --Jayron32 23:41, 12 May 2011 (UTC)
Content removed
[edit]I removed two sections that were instructions on how to analyze structures. One didn't actually seem to address the idea of hybridization at all (maybe it was just about determining valency). The other did lead from valence-electron counting to atomic hybridization, but was simplified so much that it contradicted the analysis of water and pentavalent atoms in previous sections of this article and also the N in pyrrole (and anything else with a similar resonance effect). In addition, there were WP:TONE/WP:NOT#HOWTO problems in the writing. DMacks (talk) 19:11, 4 February 2012 (UTC)
Sulfur hexafluoride
[edit]Since SF6 is a regular octahedron with six equal S-F bond lengths, I question the claim of sp hybridization in this molecule. It seems to me that if only one p orbital mixed with the s, then two bonds should be shorter than the other four. Such a bond length inequality is indeed found in PF5 for which the axial (p) are longer than the equatorial (sp2).
Is there a source for the claim of sp hybridization in SF6? It is not in Magnusson's paper which is cited in this section. Dirac66 (talk) 02:40, 8 September 2012 (UTC)
The Hypervalent molecule article describes SF6 to have 2 3c-4e bonds and 2 ordinary covalent bonds, where the ordinary covalent bonds are the ones that seem to be hybridised, judging from how in hypervalent bonding the 3c-4e bonds are always described to occupy pure p-orbitals. The equal bond lengths are explained by resonance, where all 6 bonds have equal 3c-4e, p and s character. --Officer781 (talk) 06:57, 9 September 2012 (UTC)
- This source describes the bonding in iodine heptafluoride. It's a 6c-10e bond occupying two p-orbitals, which according to the source "results in the equatorial I-F bonds being significantly longer than the axial ones" (kind of similar to PF5 in that the equatorial and axial bonds are different). By the way, I don't have proper knowledge of wikipedia citing practices, could you help cite them for me? Thanks.--Officer781 (talk) 07:00, 9 September 2012 (UTC)
- If the axially-opposite pairs of F make 3-c-4-e MOs, then SF6 sounds approximately like s + 3p (unhybridized), since each such F–S–F set either involves just an S(p) (the occupied bonding and unoccupied antibonding MOs) or does not involve S at all (the occupied nonbonding MO). The nonbonding orbitals, which have two F(p) that are in-phase at the central S, seem like they could overlap with S(s) and actually be bonding. In that case we would not have three independent F–S–F 3-c-4-e sets, since all three of these nonbonding MOs would have to be considered together with the same one S(s) orbital. If the S(s) interacts with all the ligands that bond to all three S(p), either we need to ignore all the combined interactions (partitioning the F(p)–S(p)–F(p) bonding/antibonding from the F(p)–S(s)–F(p) bonding or nonbonding) as S non-hybridized or consider them all together as S sp3? Or to put it more simply, how can we choose to hybridize with only some orthogonal p axes if all three axes have equivalent bonds? Googling for the molecular orbital diagram, I find lots of illustrations where the lowest-energy bonding orbital is "S(s) + 6F(p)" and the next few occupied ones are various combinations of S(p) with nF(p), or just nF(p) combinations themselves. DMacks (talk) 07:39, 9 September 2012 (UTC)
- hmm. I've only been taught in valence bond theory, so I do not understand the molecular orbital terminology. from this source: https://docs.google.com/viewer?a=v&q=cache:nQViZvlFDNQJ:www.stanford.edu/group/Zarelab/publinks/184.pdf+&hl=en&gl=sg&pid=bl&srcid=ADGEESjBrushBYNGJmvjLsIpdpnx-gTyLMHVOet9O0zr8Jeq3luQm3pW8yNvOru4nzi7NQtmcWqLEnkSdJDafanoiffqTTE_O2s8eDiSubEV8uic3AQ7TeyGnw4McsHYcc0T3b01Xv5V&sig=AHIEtbS9DWW8HQgvouBWSHUqSP4FZgrEFA
- it seems that the 3 3c-4e bond model seems correct. Although it doesn't seem to satisfy the octet rule because it leaves one empty s orbital.--Officer781 (talk) 07:47, 9 September 2012 (UTC)
- Either way, I feel that any mention of the purely octahedral molecules not having sp hybridisation unlike the electron pair-substituted members should be placed in a separate explanation apart from the list of shapes as that list is supposed to describe the correlation of a general shape, electron pair-substituted or not, to the hybridisation.--Officer781 (talk) 11:41, 9 September 2012 (UTC)
- The bonding in SF6 is more complicated than the simple sp hybridation in C2H2 or CO2 or BeF2. The hypervalent molecule article actually has two explanations. The Hypervalent molecule#Hexacoordinated sulfur section refers to 3c-4e bonding for each resonance structure, so that in the molecule as a whole, 3c-4e bonding is distributed across all 6 S-F bonds and there are no non-participating bonds to be sp-hybridized. The Hypervalent molecule#Bonding in hypervalent molecules section gives a more detailed MO description similar to DMacks' description above.
- I think the simple description of hypervalent molelcules as sp-hybridized should be restricted to molecules with only one axis having two axial ligands, such as XeF2 and ClF3. When there are two or three such axes, a more complex description is required.
- As for citing procedures, you can use brackets in the source code to replace the URL by a title like this. Check the source code to see what I did. For an article it only works if there is a References header at the bottom with a reflist statement; see the source code of the article for an example. Dirac66 (talk) 22:39, 9 September 2012 (UTC)
- Oops. The 3c-4e bond of XeF2 is formed by the Xe p orbital, and the 3 lone pairs are sp2 hybridized, not sp. Similarly ClF3 forms a 3c-4e bond with the Cl p orbital, and the equatorial Cl-F bond and the 2 lone pairs are roughly sp2 not sp. My error.Dirac66 (talk) 00:47, 11 September 2012 (UTC)
- I've revamped the explanation for AX6 and AX7 molecules to explain the inconsistency, and also accounts for the lack of angle deviation for ClF5. I think this should be more accurate as most atoms with large radii (usually beyond period 2) have been shown to undergo little hybridisation. You might wanna check it out to see if it makes sense.--Officer781 (talk) 07:31, 10 September 2012 (UTC)
- After googling a few papers I've realized that only the non-substituted members of the AX5, AX6 and AX7 molecules don't follow the standard description. Sorry for the wrong description and many useless edits. The entire description should be fixed now.--Officer781 (talk) 05:38, 13 September 2012 (UTC)
- It looks like the article content is back to where we started, with the uncited assertion that SF6 is sp hybridized. Resonance is actually a complete fiction as to the real electronic and physical state, it's just a convenient model that gives results that are consistent with many (but definitely not all) observations. In particular, there definitely are not three distinct states such as:
- S (2 F with normal sp sigma) (4 F as two pairs of 4e-3c using just p)
- that switch off to give a time-averaged equivalency among all the axes. It's true that each of the three resonance structures would be sp, but it's not true to say "...therefore the actual thing is" because the three sp being hybridized are different p. If you're mixing all three p with the s, you're not sp1. As Dirac says, "3c-4e bonding is distributed across all 6 S-F bonds and there are no non-participating bonds to be sp-hybridized". DMacks (talk) 14:24, 13 September 2012 (UTC)
- Unless we switch to the explanation where the s-orbital bonds with all the co-ordinating atoms separate from the p-orbital bonding? But I have no idea how to illustrate that, especially within a more valence-bond context.--Officer781 (talk) 15:07, 13 September 2012 (UTC)
- It looks like the article content is back to where we started, with the uncited assertion that SF6 is sp hybridized. Resonance is actually a complete fiction as to the real electronic and physical state, it's just a convenient model that gives results that are consistent with many (but definitely not all) observations. In particular, there definitely are not three distinct states such as:
- After googling a few papers I've realized that only the non-substituted members of the AX5, AX6 and AX7 molecules don't follow the standard description. Sorry for the wrong description and many useless edits. The entire description should be fixed now.--Officer781 (talk) 05:38, 13 September 2012 (UTC)
Partial overhaul of article
[edit]I've separated the section on misconceptions, generalized the water description to all molecules with lone pairs and created an exceptions section where those that don't follow the general scheme are listed. This is partially to decouple this page from VSEPR theory as VSEPR does not correlate to the hybridisation types as quantum chemical calculations have shown.--Officer781 (talk) 09:08, 11 November 2012 (UTC)
Hypervalent molecules expand their octets or dodectets
[edit]This section title is quite confusing. For main group molecules, chemists (like Pauling) thought a long time ago that hypervalence is due to expanded s2p6 octets. However as the article correctly says, this concept is now obsolete so why have a section title which says that it is true? Unfortunately some readers skim and will only read the section title, so let's have it say something which is true.
As for d(u)odectets, I have never seen this word (with any spelling) and it is not explained in the article. From the text, I think you mean that transition metal complexes tend to complete a d10s2 shell. But again, the title implies that p orbitals are important and the text explains that they are not, so the title is misleading.
Perhaps a better title would be Hypervalent molecules have s2p6 or s2d10 electron shells, if that is true. Dirac66 (talk) 03:59, 11 December 2012 (UTC)
- Implemented. Please do take a look and see if the titles are alright.--Officer781 (talk) 07:26, 12 December 2012 (UTC)
- As a minor note I generally don't like superscripts in level-2 headers but I don't see many reasonable alternatives.--Jasper Deng (talk) 07:50, 12 December 2012 (UTC)
- Yes, that is a much clearer header. The superscripts are not close to another line of text so I think they are all right. To say the same thing clearly using only words would require a much longer header with two or three lines, which is worse I think. Dirac66 (talk) 14:09, 12 December 2012 (UTC)
- Sorry, but I have thought about this header some more. For the main group examples it is true that molecules such as PF5 and SF6 which have been classed as hypervalent actually have an s2p6 shell with minimal d participation. Similarly TM complexes have minimal p participation according to recent calculations, but ... these complexes are not generally described as hypervalent. So I think the best solution is to remove the subsection header in question and just promote the subsubsections to subsections, one for hypervalent main-group molecules and one for TM complexes without describing them as hypervalent. Dirac66 (talk) 02:17, 15 December 2012 (UTC)
"No hybridisation"
[edit]I do not believe that section provides an adequate summary of hybridisation in hypervalent molecules, especially since it does not fully address situations where there are no lone pairs like sulfur hexafluoride.--Jasper Deng (talk) 05:44, 12 December 2012 (UTC)
- You might want to read the post "Sulfur hexafluoride" before this. We had a debate on sulfur hexafluoride before it was agreed sp3 hybridisation is the best way to explain this. You can imagine this by instead of splitting the four orbitals into four, we split it into six "partial orbitals" directed towards the ligands to accomodate the electron pairs which are partially bonding and non-bonding. Consistent with MO-theory in the sense that the "space" between the central atom and ligand consist of both the bonding orbital and non-bonding orbital.--Officer781 (talk) 07:16, 12 December 2012 (UTC)
- Would we really call this sp3? The reader generally thinks about orbital conservation - the number of orbitals mixed must produce an equal number of orbitals out. I don't find that a very satisfactory explanation using hybridisation and 3c-4e is much more intuitive to me. The octafluoroxenate anion and iodine heptafluoride then become very hard to explain using hybridisation because it's not easy to imagine 4 orbitals acommodating 7 electron pairs (perhaps 3 orbitals taking 6 is easier). More importantly, how would we draw orbital diagrams for these if we were to treat them purely using sp3 (not 3c-4e)?--Jasper Deng (talk) 07:23, 12 December 2012 (UTC)
- Nono, the sp3 talks about the hybridisation and 3c-4e talks about the details of bonding (here adapted to valence-bond terms, so might be confusing. If there is any better way to explain it without invoking MO theory, please do discuss). Iodine heptafluoride is included in the article as sp3 too as there is no lone pair (ie there are only enough pairs to put between the central atom and all the ligands). the octafluoroxenate bonds would then be purely p in character as the s-orbital holds the electron pair. But as for the sp3, I do agree it is somewhat inadequate. Any ideas for how we should call it?--Officer781 (talk) 07:28, 12 December 2012 (UTC)
- I have no idea, and it just goes to show that hypervalency discussion is not truly possible using just the orbital hybridization model, as has been established. I think we shouldn't attempt to explain it using that model, and I now think we might want to call those hypervalent molecules "Cannot be explained by hybridization" in the table heading, though that may be a little too long.--Jasper Deng (talk) 07:37, 12 December 2012 (UTC)
- Hmm. Personally I am kind of reluctant to direct the readers to MO theory as many are not trained in it. But if that's the only choice to make it simple, then maybe we should switch to it. Perhaps one active in this area like Dirac66 could share some opinions.--Officer781 (talk) 07:41, 12 December 2012 (UTC)
- d-orbital hybridization seems to be the simplest explanation to those readers but would still be confusing as it's now established to be inaccurate. Do we have alternatives?--Jasper Deng (talk) 07:48, 12 December 2012 (UTC)
- Personally I'd keep the current explanation of dividing orbitals into non-integer parts (partial-orbitals, if you like) if it's mathematically sound and reject it if it's not as it may probably be the only way to explain molecule shape using hybridisation (a hard-to-picture and non-intuitive explanation though. I've removed the details of electron count and left it to 3c-4e. We could try to simplify the explanation further). Perhaps one who knows the math of hybridisation can shed some light as hybridisation is essentially mathematical in nature, although only the concept without the math is known to most of us.--Officer781 (talk) 14:59, 13 December 2012 (UTC)
- d-orbital hybridization seems to be the simplest explanation to those readers but would still be confusing as it's now established to be inaccurate. Do we have alternatives?--Jasper Deng (talk) 07:48, 12 December 2012 (UTC)
- Hmm. Personally I am kind of reluctant to direct the readers to MO theory as many are not trained in it. But if that's the only choice to make it simple, then maybe we should switch to it. Perhaps one active in this area like Dirac66 could share some opinions.--Officer781 (talk) 07:41, 12 December 2012 (UTC)
- I have no idea, and it just goes to show that hypervalency discussion is not truly possible using just the orbital hybridization model, as has been established. I think we shouldn't attempt to explain it using that model, and I now think we might want to call those hypervalent molecules "Cannot be explained by hybridization" in the table heading, though that may be a little too long.--Jasper Deng (talk) 07:37, 12 December 2012 (UTC)
- I realized I did not answer your question on orbital diagrams. In such cases as each electron pair does not fully reside on the central atom, it would probably be impossible to do those. The molecular orbital 3c-4e diagrams would serve to illustrate how many electrons are in bonding or nonbonding orbitals. We could probably just convince readers that we can split orbitals into non-integer parts to see how the orbitals are spread to explain the shape. Electron-occupancy in specific orbitals for hypervalent molecules probably isn't the job of hybridisation to tackle, but rather 3c-4e.--Officer781 (talk) 11:10, 14 December 2012 (UTC)
- Nono, the sp3 talks about the hybridisation and 3c-4e talks about the details of bonding (here adapted to valence-bond terms, so might be confusing. If there is any better way to explain it without invoking MO theory, please do discuss). Iodine heptafluoride is included in the article as sp3 too as there is no lone pair (ie there are only enough pairs to put between the central atom and all the ligands). the octafluoroxenate bonds would then be purely p in character as the s-orbital holds the electron pair. But as for the sp3, I do agree it is somewhat inadequate. Any ideas for how we should call it?--Officer781 (talk) 07:28, 12 December 2012 (UTC)
- Would we really call this sp3? The reader generally thinks about orbital conservation - the number of orbitals mixed must produce an equal number of orbitals out. I don't find that a very satisfactory explanation using hybridisation and 3c-4e is much more intuitive to me. The octafluoroxenate anion and iodine heptafluoride then become very hard to explain using hybridisation because it's not easy to imagine 4 orbitals acommodating 7 electron pairs (perhaps 3 orbitals taking 6 is easier). More importantly, how would we draw orbital diagrams for these if we were to treat them purely using sp3 (not 3c-4e)?--Jasper Deng (talk) 07:23, 12 December 2012 (UTC)
Back to the definitions
[edit]This article and the above discussion seem confused as to the exact meaning of terms such as sp3 and sp2 especially sp. Before discussing SF6 further, we need to return to some basic definitions (which should be added to the article). The intro should specify that hybrid orbitals are linear combinations of orbitals on a given atom, introduced by Pauling as approximate description of orbitals directed towards other atoms to form bonds. And we should refrain from guessing hybridization schemes which cannot be found in reliable sources. See WP:OR.
The term sp3 does not mean just any combination of one s + three p; it implies that the weight of the s component is 1/4. The (normalized) wave function = 1/2 ψs + (31/2/2)ψpσ, where pσ is directed along the bond, or an equivalent combination of p orbitals along coordinate axes. These coefficients are fixed by symmetry for CH4, and are a good approximation for substituted methanes which also form four sigma bonds. Similarly sp2 describes an orbital which is 1/3 s, as is approximately true for C2H4 since the pi bond has no s-orbital contribution.
Molecules with lone pairs are more complex and the Pauling schemes are less exact. H2O is sometimes described as sp3, but the bond angle is less than 109.5° which implies that the AO forming the bond is less than 25% s. The true % s can be estimated from the bond angle or another physical property or from molecular orbital calculations. If for example the % s is about 20%, some describe the hybridization as sp4, which does NOT mean that 4 p AOs are involved. Similarly the sp2.5 now in the article means that the % s is 1/3.5 = 29%. An unspecified combination can be written as spx. However some (e.g. some solid-state physicists) describe this hybridation as sp, which is confusing as it suggests sp1 (50% s) as in the C2H2 molecule.
As for SF6, Pauling proposed sp3d2 which implies 17% s, 50% p, 33% d. More recent work (such as that by Magnusson cited in the article) shows that the % d is much smaller. So only 4 AOs on S are significantly populated and form 6 bonds, using 3c-4e bonds as already discussed. So what is the hybridization of the sulfur orbital which forms the 3c-4e bond on each axis? If it is called sp hybridization, that means only that there is no (significant) d participation, which is a confusing use of the term sp and to be avoided here I think. It cannot mean that the sulfur bonding orbital on each axis is 50% s as that would mean a total on the 3 axes of 150% which is impossible. As for sp3, this means 25% on each axis and 25% in a lone pair, which is possible; however the bond angle of 90° suggests pure p orbitals leaving s as a mostly non-bonding pair. So not hybridized is the simplest model, although MO calculations may indicate some small s character in the bonding MO’s. In any case we should not guess and should only include a value if we can find a source.
Finally re mention of orbitals being split into non-integer parts or “partial orbitals”. There is no such concept in quantum mechanics. If intended meaning is one (or more) lobes in space, this looks pretty in diagrams but does not correspond to a one-electron wave function which can hold two electrons. Similarly for a mathematical component of an orbital function, which also is not an electron state which can hold 2 electrons. If the term “partial orbital” has some other meaning of which I am unaware, we need a source. To describe 3c-4e bonding without talking about “partial orbitals”, just say that the orbital on the central atom can bond to 2 other atoms at once, and link to the 3c-4e article for more detail. Dirac66 (talk) 23:01, 15 December 2012 (UTC)
- Alright. I've inserted a source from GVB analysis that talks about hybrids in hypervalent molecules and removed the mention of partial orbitals. Also inserted the definitions for the spx sdx terminology and revamped the usage of the terminology accordingly. Please do help me verify the changes for quality. The next step might be to simplify the main-group-with-lone-pairs table as it seems to be too complicated. Any ideas?--Officer781 (talk) 13:19, 19 December 2012 (UTC)
- This is better. I have made a few more changes, of which the most important is to write a hybrid orbital wavefunction explicitly. I think this will be clearer, at least for readers who have seen wavefunctions before. Dirac66 (talk) 02:53, 24 December 2012 (UTC)
orbital hybridisation of hypervalent molecules
[edit]Hmm. The linear independence of hypervalent molecule hybrids is a really great revelation for the hybridisation of hypervalent molecules, I must say. I was wondering, if the F p orbitals are counted in the hybridisation as part of the linearly independent set, should we revise the name of the hybridisation to reflect that? Say, sp3x2 for SF6 where x signifies ligand orbital (nonbonding) character? Or is there terminology already given in the paper by Cooper (I don't have access to papers)?--Officer781 (talk) 03:31, 10 January 2013 (UTC)
- Wikipedia policy as per WP:OR is not to go beyond what we find in the sources, and I judge that here this means we should not invent new notation. All the literature which I know reserves the term hybridisation for the combination of orbitals centred on the same atom. For example, the paper by Cooper et al. says (for SF6) the spin-coupled orbitals (of GVB theory) which form the bonds ... "are based on six equivalent spx-like hybrids on sulfur, but each of them also has significant F(2p) participation." So the spin-coupled bond orbital includes F(2p) but the hybrid does not. This is probably what you meant by saying that the hybrids are not full orbitals or partial orbitals, although these terms are also not usually used in the literature. Dirac66 (talk) 15:27, 10 January 2013 (UTC)
- Hmm. Sorry for the comparatively late "reply", but today I found a source in a book "Pauling's Legacy: Modern Modelling of the Chemical Bond" where a section by Cooper describes, for example, a Cl-F bond in say Cl-Fn as "Cl(spx-like)+X(2p) hybrid overlapping a distorted X(2p) function", which seems adequate as an external source with a notation for such a hybridisation. It seems to me to be more appropriate as we need a proper description of pre-bond hybrids in such atoms consistent with the descriptions for non-hypervalent molecules. sp3 hybrids in total would consist of only four orbitals and cannot describe the hybrids in SF6 for example but S(sp3)+F(p) can as it would consist of six orbitals. I presume this is adequate to obey the stated Wikipedia policy?--Officer781 (talk) 14:10, 23 February 2013 (UTC)
- Interesting. I haven't seen this book, but the sentence seems to describe the bond rather than the hybrid orbital on the Cl. And the length of the phrase suggests that the author is groping to find an adequate label for a concept which is hard to describe. Certainly this notation has not become standard. We can quote the example used by Cooper in the text as a description of the bond, but I would not use this notation in the tables of hybridization types. And generalization to other examples is risky unless we are certain we have it right. For the case of SF6 there is a pσ orbital on each of the six F atoms directed toward the S, so how we can select only two to be considered bonding? Dirac66 (talk) 01:52, 24 February 2013 (UTC)
- According to the text it seems that each hybrid orbital would "borrow" the ligand character from the ligand orbital it is bonding with. That means we select a net total of two equally from all six F orbitals (I don't like this description though. I think we're talking about orbital character rather than full orbitals). I think it's more useful if we consider each hybrid individually as one complete orbital made up of a "partial" central atom orbital character and a "partial" ligand orbital character in the same way an sp3 hybrid is made up of a "partial" 1/4 s orbital character and a "partial" 3/4 p orbital character. And the text's full words are "Each Cl-X bond is comprised of a Cl(spx-like)+X(2p) hybrid overlapping a distorted X(2p) function", so I don't think he was describing the bond itself, but the hybrid the bond is comprised of.--Officer781 (talk) 07:00, 24 February 2013 (UTC)
- Hmm. It appears that part of the book is actually a paper by Cooper, see doi: 10.1016/S1380-7323(99)80022-3 --Officer781 (talk) 12:14, 24 February 2013 (UTC)
- Could you reference this paper in conventional format please - journal title, volume, pages, year? I can access many journals but not the DOI system, so the DOI numbers do not help me find the paper. Dirac66 (talk) 22:42, 24 February 2013 (UTC)
- David L. Cooper , Terry P. Cunningham , Joseph Gerratt , Mario Raimondi (1999). "Hypercoordinate bonding to main group elements: the spin-coupled point of view". Computational and Theoretical Chemistry 6: 537–553. doi:10.1016/S1380-7323(99)80022-3 --Officer781 (talk) 15:34, 25 February 2013 (UTC)
- Could you reference this paper in conventional format please - journal title, volume, pages, year? I can access many journals but not the DOI system, so the DOI numbers do not help me find the paper. Dirac66 (talk) 22:42, 24 February 2013 (UTC)
- Hmm. It appears that part of the book is actually a paper by Cooper, see doi: 10.1016/S1380-7323(99)80022-3 --Officer781 (talk) 12:14, 24 February 2013 (UTC)
- According to the text it seems that each hybrid orbital would "borrow" the ligand character from the ligand orbital it is bonding with. That means we select a net total of two equally from all six F orbitals (I don't like this description though. I think we're talking about orbital character rather than full orbitals). I think it's more useful if we consider each hybrid individually as one complete orbital made up of a "partial" central atom orbital character and a "partial" ligand orbital character in the same way an sp3 hybrid is made up of a "partial" 1/4 s orbital character and a "partial" 3/4 p orbital character. And the text's full words are "Each Cl-X bond is comprised of a Cl(spx-like)+X(2p) hybrid overlapping a distorted X(2p) function", so I don't think he was describing the bond itself, but the hybrid the bond is comprised of.--Officer781 (talk) 07:00, 24 February 2013 (UTC)
- Interesting. I haven't seen this book, but the sentence seems to describe the bond rather than the hybrid orbital on the Cl. And the length of the phrase suggests that the author is groping to find an adequate label for a concept which is hard to describe. Certainly this notation has not become standard. We can quote the example used by Cooper in the text as a description of the bond, but I would not use this notation in the tables of hybridization types. And generalization to other examples is risky unless we are certain we have it right. For the case of SF6 there is a pσ orbital on each of the six F atoms directed toward the S, so how we can select only two to be considered bonding? Dirac66 (talk) 01:52, 24 February 2013 (UTC)
- Hmm. Sorry for the comparatively late "reply", but today I found a source in a book "Pauling's Legacy: Modern Modelling of the Chemical Bond" where a section by Cooper describes, for example, a Cl-F bond in say Cl-Fn as "Cl(spx-like)+X(2p) hybrid overlapping a distorted X(2p) function", which seems adequate as an external source with a notation for such a hybridisation. It seems to me to be more appropriate as we need a proper description of pre-bond hybrids in such atoms consistent with the descriptions for non-hypervalent molecules. sp3 hybrids in total would consist of only four orbitals and cannot describe the hybrids in SF6 for example but S(sp3)+F(p) can as it would consist of six orbitals. I presume this is adequate to obey the stated Wikipedia policy?--Officer781 (talk) 14:10, 23 February 2013 (UTC)
OK thanks. My library apparently has no access to this journal, so I will assume you have understood the article correctly and try to answer as best I can. I think you have indeed found a valid source which justifies mentioning this new notation in the article. However the traditional Pauling schemes are still much more common in organic and inorganic textbooks, and it would confuse readers to suppress them entirely. So I suggest that we mention three hybridization assignments for the hypervalent molecules and explain the problem with each.
- Pauling scheme (ex. sp3d2 for SF6 plus an example for a transition metal complex). Widely used but now recognized to be invalid because d-participation is minimal for main-group central atoms (or p for transition metals).
- spx and sdx scheme (ex. sp3 for SF6) including only orbitals whose participation is significant. Problematic because there are fewer hybrids than bonds to be formed. (Hypervalence)
- Schemes including ligand orbitals as per Cooper et al. Problematic because do not fit the basic definition of a hybrid atomic orbital as an orbital on the central atom.
In short, the situation seems to be that no scheme is now considered completely satisfactory and universally accepted. As for the table, logically we should place it after this discussion and have three columns, one for each scheme. Dirac66 (talk) 00:48, 26 February 2013 (UTC)
- But I'm still not absolutely sure of this analysis though, although Cooper's wordings seem to suggest this. If you could access this (another paper by Cooper on the subject): http://link.springer.com/article/10.1007/PL00013292 maybe you could get a picture that could be combined with my incomplete one (some of the pages from the book i mentioned earlier are blocked from view in google books preview: http://books.google.com.sg/books?id=Ct6_YHZALFQC&pg=PA537&lpg=PA537&dq=chemical+bonding+to+hypercoordinate&source=bl&ots=KKMzn0r6DQ&sig=FNZ3xfV101nzFiiRj1tswa9WTZM&hl=en&sa=X&ei=kaYoUfb-BcbLrQfnq4HADA&ved=0CDEQ6AEwATgK#v=onepage&q=chemical%20bonding%20to%20hypercoordinate&f=false . Also, I have no knowledge of quantum chemistry at all, only the basic concepts taught to chemistry students, so my interpretation is likely to be very off.) to get a better idea. Meanwhile, I think I'll type a new message here that shows the table I might want to insert under the "misconceptions" part for Pauling's model as proposed for point 1. Points 2 and 3 are equivalent: we are trying to decrypt what Cooper's model is. Point 2 was just our previous attempt.--Officer781 (talk) 12:26, 26 February 2013 (UTC)
| Classification |
|
main group with lone pairs |
|---|---|---|
| Trigonal bipyramidal |
|
|
| Square bipyramidal |
|
|
| Pentagonal bipyramidal |
|
|
| Square antiprismatic |
|
- |
Would something like this work? Coincidentally, the orbitals required for hybridisation for Pauling's model is the same regardless of whether the compound is main group or transition metal (if applicable).--Officer781 (talk) 12:50, 26 February 2013 (UTC)
- I have now looked through the 1994 and the 2001 papers by Cooper et al. that you have cited. The 1999 paper is in Comput + Theo Chem which I cannot access, and Google Books gives me only fragments which are probably the same ones you see. Based on the two papers I can access, the only notation the authors use consistently are the Pauling ones such as sp3 and d2sp3. The dsp schemes are usually followed by a phrase to say there is no evidence for them. More complex descriptions resembling your "Cl(spx-like)+X(2p) hybrid overlapping a distorted X(2p) function" (which I have not yet found in the source)seem to be only mentioned occasionnally and not systematically. So this notation does not seem to be used enough to be "decrypted", and I return to my position that they should not be in the tables, but only (perhaps) in the text for the same examples as in the sources.
- As for the above table, I am having trouble understanding it. I will try again. Dirac66 (talk) 23:55, 1 March 2013 (UTC)
- The quote is from page 550 of the book. Anyway, from what Cooper says my inference is that the singly-occupied central atom orbitals as described have ligand orbital character as is what you inferred, but i simply put the ligand orbital character into a notation form similar to what Cooper has described. It's nonstandard as nobody uses it, which means that perhaps if it violates wikipedia OR, I guess we might want to either remove that part of the table or something. Don't think it's worth it to describe a superseded theory such as Pauling's "expanded octet".--Officer781 (talk) 09:53, 2 March 2013 (UTC)
- About the table, on second thought, I think for the lone pairs column the current table in the article is sufficient and so this table might be better:
- The quote is from page 550 of the book. Anyway, from what Cooper says my inference is that the singly-occupied central atom orbitals as described have ligand orbital character as is what you inferred, but i simply put the ligand orbital character into a notation form similar to what Cooper has described. It's nonstandard as nobody uses it, which means that perhaps if it violates wikipedia OR, I guess we might want to either remove that part of the table or something. Don't think it's worth it to describe a superseded theory such as Pauling's "expanded octet".--Officer781 (talk) 09:53, 2 March 2013 (UTC)
| Classification | Main group | Transition metal |
|---|---|---|
| AX2 | - |
|
| AX3 | - |
|
| AX4 | - |
|
| AX5 |
| |
| AX6 |
| |
| AX7 |
| |
| AX8 |
| |
I'm still of the opinion that we provide this table as an alternative interpretation in the misconceptions section and keep the current system as the "best compromise" in the main table (seems to reflect what Cooper et al. was trying to convey, as arrived by both you and me together-I didn't know the orbitals had ligand character) to reflect the fact that in computational chemistry circles now the molecules are not considered to utilize both p and d orbitals simultaneously. Otherwise we have no system to describe hypervalent molecule hybridisation as there are hardly any authors of papers who put forward a systematic, organized interpretation. Only the general details like what Cooper et al. did.--Officer781 (talk) 12:04, 2 March 2013 (UTC)
- This is much clearer than the previous table. For one thing, it is better to order (in the left column) by AXn than by geometry, since some readers will know the formulas but not the geometry. Also there are fewer columns and the purpose of each is clear. Dirac66 (talk) 01:43, 3 March 2013 (UTC)
- It's been a long time since I've commented on this discussion, but I would now think that we should present d-orbital hybridization with the disclaimer that experiments do not actually support it and that it's really only useful in the context of and convenience for a general chemistry course.--Jasper Deng (talk) 01:52, 3 March 2013 (UTC)
Participation of ligand orbitals
[edit]- To Dirac66: After conversing with Bduke who knows the theory very well, he explains that the orbitals are not strictly localized due to the partial-delocal nature of spin-coupled valence bond theory, which also uses the partial-delocal Coulson-Fischer function rather than a fully localized bond which resonates as per Pauling's version. If we consider the strictly local valence bond theory, there is no possible explanation for hypervalent molecules where we would have to resort to bonding-nonbonding resonance instead. The requirement that hybrid orbitals have to be "atomic" thus only exists in the strictly-localized Pauling version and there is no clash of definitions in the VB-delocal theory.--Officer781 (talk) 11:03, 7 March 2013 (UTC)
- I have now looked at your conversation here, and I note that Bduke applies his comment on the spin-coupled VB theory to all molecules: "as much in methane as it is in SFn". So this article should not suggest that the inclusion in a VB orbital of basis functions on other atoms (ligands) is unique to molecules described as hypervalent. The difference for hypervalent molecules is that the description of the molecule is less satisfactory without the ligand functions.
- The question for this article is how much to include at a level which will inform likely readers - say chemistry undergraduates. As a general editorial principle, the easier stuff should be presented first so that less knowledgeable readers can learn something from the article. So I suggest the following plan (each point as at least one section):
- Start with the classical spx hybridizations
- Geometrical consequences of hybridization (for spx hybridizations)
- Hybrids vs MO theory, with methane and/or water as examples (no hypervalence here)
- Pauling spd schemes AND why they are unsatisfactory
- Misconceptions
- Spin-coupled description (briefly) and explain that it improves the description of hypervalent molecules. Dirac66 (talk) 00:09, 8 March 2013 (UTC)
- The latest overhaul is an improvement, with one section on various aspects of hypervalent molecules, and misconceptions at the end. Also the new notation for the spin-coupled hybrids is an improvement, since you have removed the coefficients on the ligand orbitals which were probably not justified. Better to just use a + meaning that some combination is formed, as you have now done.
- A few brief suggestions for the moment:
- The phrase "Extended molecules" in chemistry usually means extended in space, as for polymers or giant molecules like diamond. This of course is not what is meant here, so it should be changed to hypervalent or something else.
- The term traditional is ok, but the tradition can be more clearly identified if we restore the mention that it was started by Pauling.
- The term spin-coupled will be strange to many. Ideally it should be linked to an article on the method but I have not found one. So I suggest linking to the most relevant of the several VB articles. I haven't yet decided which one. Dirac66 (talk) 00:03, 11 March 2013 (UTC)
- It should be Modern valence bond theory but the section on spin-coupled needs expansion. An aside on nomenclature. Joe Garratt, the inventor of the method always insisted it be called just spin-coupled. spin-coupled valence bond was a different method where a kind of configuration interaction was employed by mixing spin-coupled with functions using excited orbitals. However that method has totally dropped out of use and spin-coupled valence bond (SCVB) is beginning to be used for what was earlier just SC. --Bduke (Discussion) 01:52, 11 March 2013 (UTC)
- Thanks. The spin-coupled section of that article seems to be one paragraph (or part thereof). I have added a section title to make it easier to find, and perhaps to encourage expansion of the section. Dirac66 (talk) 02:46, 11 March 2013 (UTC)
- It should be Modern valence bond theory but the section on spin-coupled needs expansion. An aside on nomenclature. Joe Garratt, the inventor of the method always insisted it be called just spin-coupled. spin-coupled valence bond was a different method where a kind of configuration interaction was employed by mixing spin-coupled with functions using excited orbitals. However that method has totally dropped out of use and spin-coupled valence bond (SCVB) is beginning to be used for what was earlier just SC. --Bduke (Discussion) 01:52, 11 March 2013 (UTC)
Just a small suggestion
[edit]I think that this article goes on too much about how it is taught and how it is controversial and even wrong. I believe it should focus more on what hybridisation is. — Preceding unsigned comment added by 84.210.51.171 (talk) 21:29, 28 January 2014 (UTC)
An inconsistency and a question or two
[edit]Section spx and sdx terminology -- it says "For example, sp3 hybrids are formed from one s and three p orbitals. However, in all other cases, there is no such correspondence" and yet elsewhere in section sp2 hybrids it says "In sp2 hybridisation the 2s orbital is mixed with only two of the three available 2p orbitals:" which sounds like a correspondence to me.
In the section VSEPR electron domains and hybrid orbitals are different it says MO shows that the lone pairs in water aren't equivalent - OK - but do we have to switch to MO theory here to "prove it". Isn't there a VB explanation? ( I am thinking of Paulings original description involving no hybridisation - just p orbitals forming two bonds with hydrogens and positive charges on H atoms causing increase from 90°, with lone pairs in pz and s orbital )
Section Hybridisation and molecule shape - it says that tetrahedral AX4 involves sd3 hybridisation- is that 3d and 4s on manganese hybridising so wouldn't it be d3s? Or is this different from Paulings description?
Axiosaurus (talk) 16:30, 12 March 2014 (UTC)
- There is no physical difference between sd3 and d3s. Both imply hybrid orbitals formed from one s and three d orbitals, and the order of writing the orbitals is meaningless. Dirac66 (talk) 18:23, 13 March 2014 (UTC)
- The order of the orbitals in hybridisation schemes has often been used to indicate which orbitals were involved. For example, d2sp3 and sp3d2 are the most common example, the former indicating the involvemnt of 3d orbitals and the latter 4d for transition metals. Historically, at least in UK teaching this was used, and is found in more recent works such as "Descriptive Inorganic chemistry" by House and House. The use of 4d is termed outer orbital hybridisation and is part of the explanation of high spin v. low spin complexes. Using this "logic" I would therefore perceive a difference beween d3s and sd3. Axiosaurus (talk) 13:18, 16 March 2014 (UTC)
There is also the problem in MO theory for water, that while the canonical MOs do not make the "lone pairs" equivalent, transformation of the MOs to localised MOs (LMOs) can do so. Some mehtods of obtaining LMOs give equivalent lone pairs, while others do not. --Bduke (Discussion) 21:00, 12 March 2014 (UTC)
- Oh. Is there any compelling experimental evidence for equivalence or non-equivalence of the lone pairs which might point to one theory being more "correct"? Axiosaurus (talk) 14:59, 13 March 2014 (UTC)
- This is explained in the last section Hybridization theory vs. Molecular Orbital theory. For the closed-shell ground state, the two descriptions are equivalent and lead to the same total many-electron wave function, so experiment cannot distinguish them. However the ionized states observed in photoelectron spectra agree better with the delocalized-orbital description, as do the excited states observed in UV-visible spectra. Dirac66 (talk) 18:23, 13 March 2014 (UTC)
- I do not find the section you mention compelling. If LMO and VB are equivalent at some level of theory do they then predict ( incorrectly) identical lone pairs or not, as BDuke says the LMO approach may give different results depending on method. The section is silent on whether the lone pairs are predicted as identical.
- Taking a different tack, reading Pauling (1950 Nature of the chemical bond) and my old Cotton and Wilkinson 2d edition there seems to have been a shift in opinion between 1950 and 1965 regarding hybridisation- Pauling saying none (based on promotion energy not being compensated by the formation of only two O-H bonds) and C&W saying there is "a view" that there is sp3 hybridisation. Are there any referenceable calculations to support the statement that simple VB predicts sp3 hybridisation for water in contradiction of Paulings early work? Or alternatively do we invoke Bent's rule which may also point to identical lone pairs, I am not clear on whether this is the case.. My concern is that we are "damning" simple VB theory on the basis of sp3 which may just be an over simplification, a convenient teaching aid.Axiosaurus (talk) 13:18, 16 March 2014 (UTC)
- LMOs and VB are NOT equivalent at any level of theory. LMOs and VB are approximations. They are not observables. VB theory here is essentially about the O-H bonds, and in modern VB theory there will certainly be contributions from all basis functions on the O, including both s and p, as well as contributions from basis functions on the H. There will need to be a description of the other 4 valence electrons, but these could be part of the non-VB core and described in a MO way or two VB pairs which might or might not be equivalent. These are simple approximations. It is hard to say which is "best". It depends why you want to know. --Bduke (Discussion) 21:14, 16 March 2014 (UTC)
- This is explained in the last section Hybridization theory vs. Molecular Orbital theory. For the closed-shell ground state, the two descriptions are equivalent and lead to the same total many-electron wave function, so experiment cannot distinguish them. However the ionized states observed in photoelectron spectra agree better with the delocalized-orbital description, as do the excited states observed in UV-visible spectra. Dirac66 (talk) 18:23, 13 March 2014 (UTC)
what is the best title for a statement, do we need a new way to teach math — Preceding unsigned comment added by 198.52.30.180 (talk) 22:20, 4 March 2015 (UTC)
Two sources are often better than one
[edit]I see that Officer781 has been cleaning up some of the references, which is generally good. However I disagree with the deletion of Laing's paper as a source with the edit summary we don't need two sources referring to the same thing. There can be several reasons why two sources for a given fact can be better than one:
- Reliability: if two authors (or groups of authors) agree on a fact, it is more likely to be correct.
- Clarity: it may be easier to understand an explanation on reading two different versions
- Access: some readers may not have access to one version; citing two increases the chance that a reader may be able to find one source
- Link rot: If one of the two sources disappears from the Internet in 1 or 3 or 10 years, it is useful to have another already available.
Of course we very often only have one source because no one has bothered to find a second. But if we have two, as is the case here, it makes more sense to include both for the above reasons. So I will restore the Laing paper as a source, and retain the Weinhold group paper also. Dirac66 (talk) 00:57, 18 March 2015 (UTC)
AXE notation
[edit]I readded the "AXE notation"; I found it used in both Housecraft's "Inorganic chemistry" and "Molecular orbitals of transition metal complexes" by Yves Jean. Christian75 (talk) 10:07, 1 April 2015 (UTC)
Draft new section on hybridization of heavier p block elements
[edit]Below is a draft of a section on hybridization of heavier p block elements. It doesn't really fit into the article as written whose focus is on ideal orthogonal "first order" hybridization which describes carbon very well but the rest of the periodic table less so. Adding this in to the article as it stands would require some re-structuring. Comments?
Hybridization of s and p orbitals to form effective sp hybrid orbitals requires that they have comparable radial extent. While 2p orbitals are on average less than 10% larger than 2s, in part attributable to the lack of a radial node in 2p orbitals, 3p orbitals which have one radial node, exceed the 3s orbitals by 20-33%.[1] The difference in extent of s and p orbitals increases further down a group. The hybridization in of atoms in chemical bonds can be analyzed by considering localized molecular orbitals, for example using natural localized molecular orbitals in a natural bond orbital (NBO) scheme. In methane, CH4, the calculated p/s ratio is approximately 3 consistent with "ideal" sp3 hybridization, whereas for silane, SiH4, the p/s ratio is closer to 2. A similar trend is seen for the other 2p elements. Substitution of fluorine for hydrogen further decreases the p/s ratio.[2]The 2p elements exhibit near ideal hybridization with orthogonal hybrid orbitals. For heavier p block elements this assumption of orthogonality cannot be justified. These deviations from the ideal hybridization were termed hybridization defects by Kutzelnigg.[3]
- ^ Kaupp, Martin (2007). "The role of radial nodes of atomic orbitals for chemical bonding and the periodic table". Journal of Computational Chemistry. 28 (1): 320–325. doi:10.1002/jcc.20522. ISSN 0192-8651.
- ^ Kaupp, Martin (2014) [1st. Pub. 2014]. "Chapter 1: Chemical bonding of main group elements". In Frenking, Gernod; Shaik, Sason (eds.). The Chemical Bond. Wiley-VCH. ISBN 978-1-234-56789-7.
{{cite book}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ "Orthogonal and non-orthogonal hybrids". Journal of Molecular Structure: THEOCHEM. 169: 403–419. August 1988. doi:10.1016/j.ccr.2010.04.011.
{{cite journal}}: Unknown parameter|authors=ignored (help) – via ScienceDirect (Subscription may be required or content may be available in libraries.)
Axiosaurus (talk) 14:01, 22 April 2015 (UTC)
- If we mention nonorthogonal hybrids, we should add that this implies bonds with enough ionic character to satisfy the Pauli principle. For SiH4 as an example, if we had four pure covalent bonds with sp2 hybrids, there would be 8/3 s electrons on Si. The molecule avoids this catastrophe by shifting at least 1/6 s electron per bond off Si onto the 4 H.
- Also, I think the whole topic of nonorthogonality may be too mathematical for this article, and might be better placed in the more specialized variable hybridization article. Dirac66 (talk) 20:04, 22 April 2015 (UTC)
- a few quick comments before I pack- non-orthogonal means that there is overlap between hybrids. Variable hybridisation as that article stands is about what I would refer to as isovalent or second order hybridization - i.e. orthogonal hybrids with non classical proportions e.g. sp4, sp5 etc - these are where Bents rules apply. Perhaps what should be in this article is that overlap between s/p orbitals is necessary for production of effective orthogonal hybrids and that this criterion is only met in first row p group I will be away for a week or so. Axiosaurus (talk) 10:12, 23 April 2015 (UTC)
- I've added the proposed section to the article as it's been some time. As most of the article is based on the assumption of ideal hybridisation, I've added the proposed section towards the end of the article (logic follows first-order, second-order, deviations). I'm not exactly sure what a better layout would look like, but please do re-format the article as you deem necessary.--Officer781 (talk) 01:21, 16 June 2015 (UTC)
hybridization and electronic configuration..
[edit]How will we be able to describe or find hybridization of certain compound like C2H4,C2H6,C2H2 by using electron configuration alone?without use of orbital diagram. Alishasapkota (talk) 12:03, 2 July 2016 (UTC)
- It's fairly straightforward for normal, textbook molecules like ethane, ethylene and acetylene that you mention. If a hydrocarbon has only single bonds, its C atoms will all be sp3 hybridised. If there is a C=C double bond, the double-bonded carbons will each be sp2 hybridised. If there's a C≡C triple bond, the triple-bonded carbons will each be sp hybridised. In allene, H2C=C=CH2, the terminal CH2 carbons are sp2 and the central C atom is sp hybridised. All you really need is a Lewis structure of the molecule. --Ben (talk) 16:57, 3 July 2016 (UTC)
Basic article standards
[edit]If something is not mentioned in the body of the article, it should not be mentioned in the lead section. Also, there should not be blue links in bold face in the article lead. Seems that at least two editors are not aware of these basic standards. 95.145.130.4 (talk) 21:40, 4 May 2017 (UTC)
- Neither MOS not content-organization guidelines are good reasons for removing content that is itself supported by content guidelines. Perhaps you can figure out an alternate wording, formatting, or organization that you feel is better but that does not wind up actually losing the material itself. DMacks (talk) 00:11, 5 May 2017 (UTC)
- why do you think that what I removed should not have been removed? 95.145.130.4 (talk) 00:20, 5 May 2017 (UTC) and please confirm (as one editor clearly did not) that you understand what my edit actually changed. 95.145.130.4 (talk) 00:22, 5 May 2017 (UTC)
- @DMacks: The reason why I chose not to respond to this thread is that this IP seems (to me) to be blatant block evasion: Wikipedia:Sockpuppet investigations/200.120.158.78.--Jasper Deng (talk) 00:24, 5 May 2017 (UTC)
- pathetic. 95.145.130.4 (talk) 00:42, 5 May 2017 (UTC)
- Good eye, Jasper. CU has handled it...nothing further to do here. DMacks (talk) 01:08, 5 May 2017 (UTC)
- pathetic. 95.145.130.4 (talk) 00:42, 5 May 2017 (UTC)
Bond angles
[edit]@Dirac66: I noticed that the square planar hybridisation angle was changed from 109.5 and 180 to 90. I placed the interorbital angles in the hybridisation row because it reflects the ideal interorbital angle calculated by the formula instead of the actual molecular angle (109.5 comes from the positive form of the formula while 180 comes from the negative form). That's why 70.5 is included together with 109.5 for the sd3 tetrahedral even though it is not present in the final molecule. Also, trigonal pyramidal and trigonal prismatic complexes frequently have larger bond angles than the ideal orbital angle because of ligand repulsion. Thoughts?--Officer781 (talk) 09:39, 14 May 2020 (UTC)
- Update: I decided to include both actual bond angles and ideal orbital angles. Shapes without included angles means that the bond angle can vary. Would this be good?--Officer781 (talk) 09:50, 14 May 2020 (UTC)
- @Officer781: My concern is that the significance of your "ideal" bond angles is not clear when they disagree with the actual angles given in most textbooks. Many books agree that sp2d applies to square planar complexes. For example see the table of hybridization schemes in Housecroft and Sharpe, Inorganic Chemistry (2nd ed. p.556 table). From elementary geometry, these square planar complexes have a 90° angle between adjacent bonds and not 109° which is the tetrahedral bond angle. (Some authors but not all also include angles between 2 axial bonds, such as 180° square planar complexes.)
- I have now looked at the pages of Weinhold and Landis which you have cited. I found the formula cited for the interbond angle in spxdy (or spλdμ per the authors) hybridization as equation 4.27 on p.375, and checked that it does seem to give 109° and 180° for x=2, y=1. But I also note that Weinhold and Landis do not actually specify the bond angles for sp2d or for sp3d, but only for sp3d2 (on p.376) where everyone agrees that the bond angle is 90° (and 180° between nonadjacent bonds). Also for the case of sp3d1 hybridization also, the equation seems to give one real-valued angle (123.4°) and one complex-valued angle (with a cosine greater than one), whereas chemistry books give a trigonal bipyramidal shape (in PF5 for example) with angles of 90° and 120° (and 180°). Something is wrong with my (and your?) understanding of this formula.
- So the statement that the bond angle in spxdy complexes is ideally 109° is what Wikipedia calls "original research" and may be based on some misinterpretation of the Weinhold-Landis paper concerning the applicability of their equation. The third sentence of WP:OR says that "This includes any analysis or synthesis of published material that serves to reach or imply a conclusion not stated by the sources." So I think the 109° value for sp2d should be removed, unless you can find it specifically stated, hopefully with an explanation. Dirac66 (talk) 20:20, 15 May 2020 (UTC)
- Actually this formula was also given by Pauling, see [2]. I will remove the 109° for square planar (as well as the tetrahedral 70.5°). I presume these are the "best" angles for the given hybrid orbital composition with the highest overlap because they correspond to the "notches" or asymptotes of the orbital (d-containing hybrid orbitals have two notches which give rise to two answers). I remember Weinhold and Landis explaining this somewhere in the book with a picture to illustrate. sdx oritals have symmetrical notches so they are equally distant from 90°, while spxdy orbitals have asymmetrical notches due to the p-character. I presume that means that the actual sp2d hybridisation and so on actually has some kind of strain.--Officer781 (talk) 01:34, 16 May 2020 (UTC)
- Apologies, I am not able to access Inorganic Chemistry. There does not seem to be a google books preview and I am not able to go to my university library due to lockdown. I might take a look when I am able to.--Officer781 (talk) 06:36, 16 May 2020 (UTC)
Methylene sp3 hybridization
[edit]Why is methylene mentioned under the header sp3? I was trying to close out the discrepancy to remove the {{contradictory}} tag, but the alt-text is kind of a confusing run-on sentence that mentions a lot of things that aren't there, e.g., the bond angle of carbene. Is the use of the unfavorable structure useful at all as part of the explanation? Reconrabbit (talk|edits) 14:44, 20 December 2023 (UTC)
@Reconrabbit: I have now clarified this to distinguish between the hypothetical non-hybridized 90° molecule and the real hybridized 102° molecule. And I have placed the alt-text in the article where it is more legible. Dirac66 (talk) 17:36, 16 March 2024 (UTC)
- Great clarification, Thank you! It helps to make that diagram more useful in that part. Reconrabbit 20:41, 16 March 2024 (UTC)
unit vector
[edit]how to calculate unit vector 137.196.0.34 (talk) 06:43, 16 April 2024 (UTC)
- See the article on unit vector. I don't see the term used in this article. Dirac66 (talk) 14:11, 16 April 2024 (UTC)
- Wikipedia articles that use British English
- C-Class vital articles
- Wikipedia level-5 vital articles
- Wikipedia vital articles in Physical sciences
- C-Class level-5 vital articles
- Wikipedia level-5 vital articles in Physical sciences
- C-Class vital articles in Physical sciences
- C-Class Chemistry articles
- High-importance Chemistry articles
- WikiProject Chemistry articles


