Trypanosoma
| Trypanosoma | |
|---|---|
| |
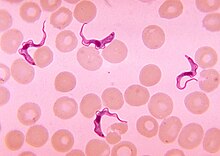
| Trypanosoma sp. among red blood cells. | |
| Scientific classification | |
| Domain: | Eukaryota |
| Phylum: | Euglenozoa |
| Class: | Kinetoplastea |
| Order: | Trypanosomatida |
| Family: | Trypanosomatidae |
| Genus: | Trypanosoma Gruby, 1843 |
| Subgenera | |
| |
| Synonyms | |
| |
Trypanosoma is a genus of kinetoplastids (class Trypanosomatidae[1]), a monophyletic[2] group of unicellular parasitic flagellate protozoa. Trypanosoma is part of the phylum Euglenozoa.[3] The name is derived from the Greek trypano- (borer) and soma (body) because of their corkscrew-like motion. Most trypanosomes are heteroxenous (requiring more than one obligatory host to complete life cycle) and most are transmitted via a vector. The majority of species are transmitted by blood-feeding invertebrates, but there are different mechanisms among the varying species. Trypanosoma equiperdum is spread between horses and other equine species by sexual contact. They are generally found in the intestine of their invertebrate host, but normally occupy the bloodstream or an intracellular environment in the vertebrate host.
Trypanosomes infect a variety of hosts and cause various diseases, including the fatal human diseases sleeping sickness, caused by Trypanosoma brucei, and Chagas disease, caused by Trypanosoma cruzi.
The mitochondrial genome of the Trypanosoma, as well as of other kinetoplastids, known as the kinetoplast, is made up of a highly complex series of catenated circles and minicircles and requires a cohort of proteins for organisation during cell division.
History
[edit]In 1841, Gabriel Valentin found flagellates that today are included in Trypanoplasma in the blood of trout.[4][5]
The genus (T. sanguinis) was named by Gruby in 1843, after parasites in the blood of frogs.[6]
In 1903, David Bruce identified the protozoan parasite and the tsetse fly vector of African trypanosomiasis.[7]
Taxonomy
[edit]A number of different methods demonstrate that the traditional Trypanosoma genus is not monophyletic, with the biflagellate Bodonida nested within. The American and African trypanosomes constitute distinct clades, implying that the major human disease agents T. cruzi (cause of Chagas’ disease) and T. brucei (cause of African sleeping sickness) are not closely related to each other.[8]
Phylogenetic analyses suggest an ancient split between a branch containing all Salivarian trypanosomes and a branch containing all non-Salivarian lineages. The latter branch in turn splits into a clade containing bird, reptilian and the Stercorarian trypanosomes infecting mammals, and a clade with a branch of fish trypanosomes and a branch of reptilian or amphibian lineages.[9]
Salivarians are trypanosomes of the subgenera of Duttonella, Trypanozoon, Pycnomonas and Nannomonas, which are passed to the vertebrate recipient in the saliva of the tsetse fly (Glossina spp.).[10] Antigenic variation is a characteristic shared by the Salivaria, which has been particularly well-studied in T. brucei.[11] The Trypanozoon subgenus contains the species Trypanosoma brucei, T. rhodesiense and T. equiperdum. The subgenus Duttonella contains the species T. vivax. Nannomonas contains T. congolense.[12]
Stercorians are trypanosomes passed to the recipient in the feces of insects from the subfamily Triatominae (most importantly Triatoma infestans).[13] This group includes Trypanosoma cruzi, T. lewisi, T. melophagium, T. nabiasi, T. rangeli, T. theileri, T. theodori.[14] The subgenus Herpetosoma contains the species T. lewisi.
The subgenus Schizotrypanum contains T. cruzi[12] and a number of bat trypanosomes. The bat species include Trypanosoma cruzi marinkellei, Trypanosoma dionisii, Trypanosoma erneyi, Trypanosoma livingstonei and Trypanosoma wauwau. Other related species include Trypanosoma conorhini and Trypanosoma rangeli.[citation needed]
Evolution
[edit]The ancestor of modern trypanosomes absorbed a green alga around one billion years ago and co-opted some of its genetic material. This has resulted in modern trypanosomes such as T. brucei containing essential genes for the breakdown of sugars that are most closely related to plants. This difference may be used as the target of therapies.[15]
The relationships between the species have not been worked out to date. It has been suggested that T. evansi arose from a clone of T. equiperdum which lost its maxicircles.[16] It has also been proposed that T. evansi should be classified as a subspecies of T. brucei.[17]
It has been shown that T. equiperdum has emerged at least once in Eastern Africa and T. evansi at two independent occasions in Western Africa.[18]
Selected species
[edit]Species of Trypanosoma include the following:
- T. ambystomae. in amphibians
- T. antiquus, extinct (Fossil in Miocene amber)
- T. avium, which infects birds and blackflies
- T. bennetti, which infects birds and biting midges
- T. boissoni, in elasmobranch
- T. brucei, which causes sleeping sickness in humans and nagana in cattle
- T. cruzi, which causes Chagas disease in humans
- Trypanosoma culicavium, which infects birds and mosquitoes
- T. congolense, which causes nagana in ruminant livestock, horses and a wide range of wildlife
- T. equinum, in South American horses, transmitted via Tabanidae,
- T. equiperdum, which causes dourine or covering sickness in horses and other Equidae, it can be spread through coitus.
- T. evansi, which causes one form of the disease surra in certain animals including camels[19] (a single case report of human infection in 2005 in India[20] was successfully treated with suramin[21])
- T. everetti, in birds
- T. hosei, in amphibians
- T. irwini, in koalas
- T. lewisi, in rats
- T. melophagium, in sheep, transmitted via Melophagus ovinus
- T. parroti, in amphibians
- T. percae, in the species Perca fluviatilis
- T. phedinae
- T. rangeli, believed to be nonpathogenic to humans
- T. rotatorium, in amphibians
- T. rugosae, in amphibians
- T. sergenti, in amphibians
- T. simiae, which causes nagana in pigs. Its main reservoirs are warthogs and bush pigs
- T. sinipercae, in fishes
- T. suis, which causes a different form of surra
- T. theileri, a large trypanosome infecting ruminants and transmitted by a variety of vectors including tabanids and mosquitoes
- T. thomasbancrofti, an avian trypanosome with culicine mosquito vector
- T. triglae, in marine teleosts
- T. tungarae, in frogs[22]
- T. vivax, which causes the disease nagana, mainly in West Africa, although it has spread to South America[23]
Hosts, life cycle and morphologies
[edit]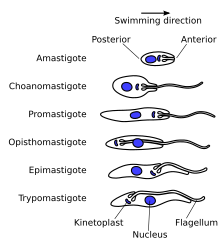
Two different types of trypanosomes exist, and their life cycles are different, the salivarian species and the stercorarian species.[citation needed]
Stercorarian trypanosomes infect insects, most often the triatomid kissing bug, by developing in the posterior gut followed by release into the feces and subsequent depositing on the skin of the vertebrate host. The organism then penetrates and can disseminate throughout the body. Insects become infected when taking a blood meal.[citation needed]
Salivarian trypanosomes develop in the anterior gut of insects, most importantly the Tsetse fly, and infective organisms are inoculated into the host by the insect bite before it feeds.[citation needed]
As trypanosomes progress through their life cycle they undergo a series of morphological changes as is typical of trypanosomatids. The life cycle often consists of the trypomastigote form in the vertebrate host and the trypomastigote or promastigote form in the gut of the invertebrate host. Intracellular lifecycle stages are normally found in the amastigote form. The trypomastigote morphology is unique to species in the genus Trypanosoma.[citation needed]
Meiosis
[edit]Evidence has been obtained for meiosis in T. cruzi, and for genetic exchange.[24] T. brucei is able to undergo meiosis within the salivary glands of its tsetse fly host, and meiosis is considered to be an intrinsic part of the T. brucei developmental cycle.[25][26] An adaptive benefit of meiosis for T. crucei and T. brucei may be the recombinational repair of DNA damages that are acquired in the hostile environment of their respective hosts.[27]
References
[edit]- ^ "WHO - The parasite". WHO. Archived from the original on September 29, 2016. Retrieved 8 March 2019.
- ^ Hamilton PB, Stevens JR, Gaunt MW, Gidley J, Gibson WC (2004). "Trypanosomes are monophyletic: evidence from genes for glyceraldehyde phosphate dehydrogenase and small subunit ribosomal RNA". Int. J. Parasitol. 34 (12): 1393–404. doi:10.1016/j.ijpara.2004.08.011. PMID 15542100.
- ^ "Taxonomy of African Trypanosoma species". msu.edu. Retrieved 2019-03-28.
- ^ Leadbeater, B.S.C & McCready, S.M.M. (2000). The Flagellates. Unity, diversity and evolution. Ed.: Barry S. C. Leadbeater and J. C. Green Taylor and Francis, London, p. 12.
- ^ Valentin, G. 1841. Ueber ein Entozoon im Blute von Salmo fario. Müller's Archiv, p. 435.
- ^ Gruby, D. 1843. Recherches et observations sur une nouvelle espéce d'haematozoaire, Trypanosoma sanguinis. Comptes Rendus de l'Académie des Sciences, 17: 1134–1136, [1].
- ^ Ellis, H. (March 2006). "Sir David Bruce, a pioneer of tropical medicine". British Journal of Hospital Medicine. 67 (3): 158. doi:10.12968/hmed.2006.67.3.20624. PMID 16562450.
- ^ Environmental kinetoplastid-like 18S rRNA sequences and phylogenetic relationships among Trypanosomatidae: Paraphyly of the genus Trypanosoma. Helen Piontkivska and Austin L. Hughes, Molecular and Biochemical Parasitology, November 2005, Volume 144, Issue 1, Pages 94–99, doi:10.1016/j.molbiopara.2005.08.007
- ^ The molecular phylogeny of trypanosomes: evidence for an early divergence of the Salivaria. Jochen Haag, Colm O'hUigin and Peter Overath, Molecular and Biochemical Parasitology, 1 March 1998, Volume 91, Issue 1, Pages 37–49, doi:10.1016/S0166-6851(97)00185-0
- ^ "salivarian". Retrieved 8 March 2019 – via The Free Dictionary.
- ^ Sex and evolution in trypanosomes. Wendy Gibson, International Journal for Parasitology, 1 May 2001, Volume 31, Issues 5–6, Pages 643–647, doi:10.1016/S0020-7519(01)00138-2
- ^ a b Dihydrofolate reductases within the genus Trypanosoma. J.J. Jaffe, J.J. McCormack Jr and W.E. Gutteridge, Experimental Parasitology, 1969, Volume 25, Pages 311–318, doi:10.1016/0014-4894(69)90076-9
- ^ Prevention, CDC-Centers for Disease Control and (2 May 2017). "CDC - Chagas Disease - General Information". www.cdc.gov. Retrieved 8 March 2019.
- ^ "Stercoraria". Retrieved 8 March 2019 – via The Free Dictionary.
- ^ Whitfield, John (2003). "Sleeping sickness bug swallowed a plant". Nature. doi:10.1038/news030127-3. Retrieved 4 October 2021.
- ^ Brun R, Hecker H, Lun ZR (1998) Trypanosoma evansi and T. equiperdum: distribution, biology, treatment and phylogenetic relationship (a review). Vet Parasitol 79(2):95-107
- ^ Carnes J, Anupama A, Balmer O, Jackson A, Lewis M, Brown R, Cestari I, Desquesnes M, Gendrin C, Hertz-Fowler C, Imamura H, Ivens A, Kořený L, Lai DH, MacLeod A, McDermott SM, Merritt C, Monnerat S, Moon W, Myler P, Phan I, Ramasamy G, Sivam D, Lun ZR, Lukeš J, Stuart K, Schnaufer A (2015) Genome and phylogenetic analyses of Trypanosoma evansi reveal extensive similarity to T. brucei and multiple independent origins for dyskinetoplasty. PLoS Negl Trop Dis 9(1):e3404. doi: 10.1371/journal.pntd.0003404
- ^ Cuypers B, Van den Broeck F, Van Reet N, Meehan CJ, Cauchard J, Wilkes JM, Claes F, Goddeeris B, Birhanu H, Dujardin JC, Laukens K, Büscher P, Deborggraeve S (2017) Genome-wide SNP analysis reveals distinct origins of Trypanosoma evansi and Trypanosoma equiperdum. Genome Biol Evol doi: 10.1093/gbe/evx102
- ^ Sazmand, Alireza; Joachim, Anja (2017). "Parasitic diseases of camels in Iran (1931–2017) – a literature review". Parasite. 24. EDP Sciences: 1–15. doi:10.1051/parasite/2017024. ISSN 1776-1042. PMC 5479402. PMID 28617666. S2CID 13783061. Article Number 21. p. 2
- ^ World Health, Organization (2005). "A new form of human trypanosomiasis in India. Description of the first human case in the world caused by Trypanosoma evansi". Wkly. Epidemiol. Rec. 80 (7): 62–3. PMID 15771199.
- ^ Joshi PP, Chaudhari A, Shegokar VR, et al. (2006). "Treatment and follow-up of the first case of human trypanosomiasis caused by Trypanosoma evansi in India". Trans. R. Soc. Trop. Med. Hyg. 100 (10): 989–91. doi:10.1016/j.trstmh.2005.11.003. PMID 16455122.
- ^ Bernal XE, Pinto CM (2016), "Sexual differences in prevalence of a new species of trypanosome infecting túngara frogs", Int J Parasitol Parasites Wildl, 5 (1): 40–47, Bibcode:2016IJPPW...5...40B, doi:10.1016/j.ijppaw.2016.01.005, PMC 4781969, PMID 26977404
- ^ Batista JS, Rodrigues CM, García HA, Bezerra FS, Olinda RG, Teixeira MM, Soto-Blanco B (2011). "Association of Trypanosoma vivax in extracellular sites with central nervous system lesions and changes in cerebrospinal fluid in experimentally infected goats". Veterinary Research. 42 (63): 1–7. doi:10.1186/1297-9716-42-63. PMC 3105954. PMID 21569364.
- ^ Messenger LA, Miles MA (2015). "Evidence and importance of genetic exchange among field populations of Trypanosoma cruzi". Acta Trop. 151: 150–5. doi:10.1016/j.actatropica.2015.05.007. PMC 4644990. PMID 26188331.
- ^ Peacock L, Ferris V, Sharma R, Sunter J, Bailey M, Carrington M, Gibson W (2011). "Identification of the meiotic life cycle stage of Trypanosoma brucei in the tsetse fly" (PDF). Proc. Natl. Acad. Sci. U.S.A. 108 (9): 3671–6. Bibcode:2011PNAS..108.3671P. doi:10.1073/pnas.1019423108. PMC 3048101. PMID 21321215.
- ^ Gibson W (2015). "Liaisons dangereuses: sexual recombination among pathogenic trypanosomes". Res. Microbiol. 166 (6): 459–66. doi:10.1016/j.resmic.2015.05.005. hdl:1983/1ecb5cba-da25-4e93-a3cb-b00a0477cb23. PMID 26027775. S2CID 9594154.
- ^ Bernstein H, Bernstein C, Michod RE (2018). Sex in microbial pathogens. Infection, Genetics and Evolution volume 57, pages 8-25. https://doi.org/10.1016/j.meegid.2017.10.024
External links
[edit]- Trypanosoma reviewed and published by Wikivet, accessed 08/10/2011.
- Trykipedia, Trypanosomatid specific ontologies
- Tree of Life: Trypanosoma
